Licence: Public Domain Mark
Credit: The medical student's manual of chemistry. Source: Wellcome Collection.
Provider: This material has been provided by the Gerstein Science Information Centre at the University of Toronto, through the Medical Heritage Library. The original may be consulted at the Gerstein Science Information Centre, University of Toronto.
29/586 (page 9)
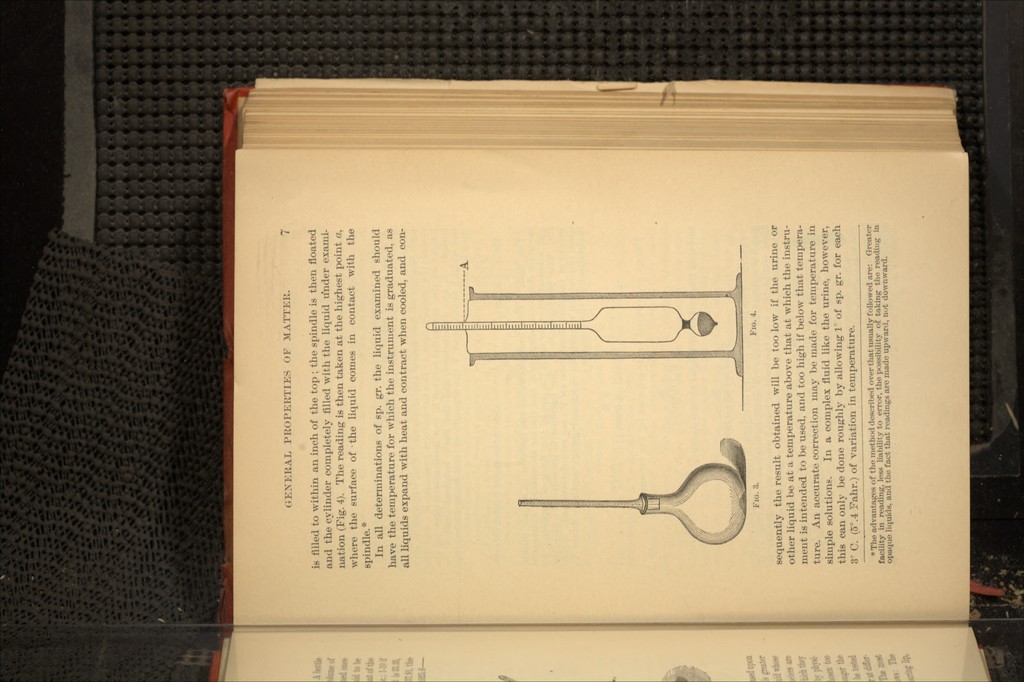
![boiling, and the mercury ceases to escape from the small tube, the barometric pressure and the temperature of the air are observed. After the apparatus is cooled, the tube (Fig. 6), with its contents is weighed,-and the difference in the level of mercury which existed in the two branches during the heating determined by breaking the capillary point, tilting the apparatus until the smaller branch is completely filled, marking the level of mercury in the larger branch, and afterward measuring the distance from that point to the opening. By the above process the following factors are determined : P=weight of substance ; T=boiling-point of external liquid ; Z=temperature of air; H=barometric pressure reduced to 0; h=difference in level of mercury in two branches of tube ; ./V=tension of vapor of mercury at T ; a=weight of mercury used ; <?=weight of mercury required to fill the tube Fig. 5 ; r=weight of mercury remaining in the apparatus after heating. From these the specific gravity, air = 1, is obtained by the •equation : B_ P 760 (1+0.00367 T) 13.59 ~ (H+h+h') 0.0012932 \(a+q) •{ 1+0.0000303 (T—t) }- —r •{ 1+ 0.00018 (T—t) H [1+0.00018 t] The sp. gr. in terms of air=l may be reduced to sp. gr. referred to hydrogen=2, by dividing by 0.06927. States of Matter.—Matter exists in one of three states; solid, liquid, and gaseous. In the solid form, the particles of matter are comparatively close together, and are separated with more difficulty than are those of liquid or gaseous matter ; or in other words the cohesion of solid matter is greater than that of the other two forms. In the liquid, the particles are less firmly bound together, and are capable of freer motion about one an- other. In the gas, the mutual attraction of the particles disap- pears entirely, and their distance from each other depends upon the pressure to which the gas is subjected. The term fluid applies to both liquids and gases, the former beiiiy; designated as incompressible, from the very slight degree to which their volume can be reduced by pressure. The gases are designated as compressible fluids, from the fact that their volume can be reduced by pressure, to an extent limited only by their passage into the liquid form. It 's highly probable that all substances, which are not decom- posed when heated, are capable of existing in the three forms of solid, liquid, and gas. There are, however, some substances which are only known in two forms—as alcohol; or in a single](https://iiif.wellcomecollection.org/image/b20996901_0029.jp2/full/800%2C/0/default.jpg)