Volume 1
Elements of chemistry : theoretical and practical / by William Allen Miller.
- Miller, William Allen, 1817-1870.
- Date:
- 1860-1862
Licence: Public Domain Mark
Credit: Elements of chemistry : theoretical and practical / by William Allen Miller. Source: Wellcome Collection.
473/510 (page 451)
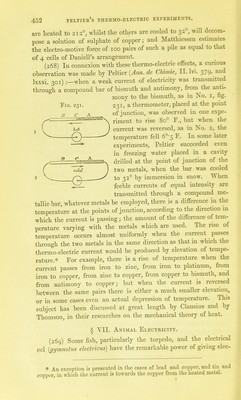
![IS increased. Sueh a battery will decompose a solution of iodide and Botto states that with a pile consisting of loo pairs of platinum and iron wire, each i inch long and -pj-^th of an inch in diameter, he succeeded in decomposing even dHuted sulphuric acid. A thermo-electric current from a single pair is sufficient to convulse the limbs of a frog. The principle of the arrangement by which a thermo-multiplier or thermo-electric bat- teiy may be constructed is shown in fig. 230; to one series of junctions, a high tempera- Fig. 230. ture, to the other a low temperature may be applied; the shaded bars, a, represent bars of antimony, those in outline, b, indicate bars of bismuth. The intensity of such a current, ^ however, is comparatively feeble, and the re- sistance which it experiences in traversing even metallic conductors of considerable dia- meter, such as the metallic bars themselves which are used in the construction of the bat- tery, seriously reduces its power. A very cheap and eflPective thermo-electric pile may be made of wires of iron and German silver. Nobili and ]\Ielloni applied a tbermo-electric battery, consisting of 36 pairs of small bars of bismuth and antimony, to thermometric purposes. Such a battery was employed by Mellon!, in his inves- tigations on radiant heat, to the exclusion of almost every other thermoscopic means. When the alternate junctions of the bars at each end of the pile were covered with lampblack, a coating was obtained Avhich absorbed the radiations proceeding from a surface the temperature of which was much below that of the human body ; even the amount of radiant heat emitted by insects could be esti- mated by connecting this battery with a galvanometer of extreme sensitiveness.* The conducting power of red phosphorus and of selenium is so slight that neither of them can be used for the construction of thermo- electric piles: that of tellurium is also small, but it is sufficient to admit of its use, and its electromotive power, when opposed thermo-electrically to bismuth, is so great that a pile con- sisting of 8 pairs of these elements, where the alternate junctions * jVIelloni used a galvanometer formed of copper wire of an inch in diameter, about 8 yards long, and arranged around the astatic needles in 40 convolutions. Much of the sensitiveness of the instrument depends upon the exact equality of the magnetization of the two needles: the compound needle should require from 55 to 60 seconds in order to complete an oscillation. G G 2](https://iiif.wellcomecollection.org/image/b28083982_0001_0473.jp2/full/800%2C/0/default.jpg)