Handbook of the polariscope and its pracitcal applications / adapted from the German editon of H. Landolt, by D.C. Robb and V.H. Veley.
- Hans Heinrich Landolt
- Date:
- 1882
Licence: Public Domain Mark
Credit: Handbook of the polariscope and its pracitcal applications / adapted from the German editon of H. Landolt, by D.C. Robb and V.H. Veley. Source: Wellcome Collection.
52/320 (page 32)
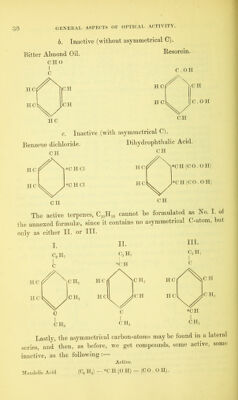
![and, consequently, the chemical constitution of such compounds, agree with those of the natural substances, a difference is never- theless found to exist in their optical properties. Direct synthesis of substances from inactive components has hitherto resulted m t e production of inactive modifications only. As already indicated, this inactivity of artificial substances may be apparent (i.e. latent) only and dependent on the following causes, which at the same time indicate the means to its possible removal 1. In synthesis, it is probable that an equal number ot dextro-rotatory and lmvo-rotatory molecules may always be forme , which mix or combine together and so produce optical neutrality. As an instance of the kind, Jungfleisch1 has shown that by converting ethylene—through the intermediate products ethylene bromide, ethylene cyanide, succinic acid, and dibromo-succinic acid—into tartaric acid, the inactive form is obtained, which by crystallization ot its sodium-ammonium salt may be separated into dextro-tartanc an lsevo-tartaric acids. This, at present, is the only instance that can be alleged of an artificial active substance; and even here it mus be observed that the direct result of the synthesis, the para-tartano acid, exhibits no optical power.3 As the physical and chemical properties of such opposite-rotating modifications may differ hut little, they cannot in general he separated without great difficulty, and the more so, when they form not merely mechanical mixtures but true chemical combinations, as is the case with para-tartaric acid. _ Indeed the only substance as yet, whose inactivity depends on neutralization, which has been separated into right- and left-rotatmg modifications is para-tartaric acid. The separation can be effected by one of the following methods :— a. By crystallization of the sodium-ammonium salt, and separa- tion by selection of the crystals with dextro-hemihedric from those with kevo-hemihedric planes (Pasteur8). This method has been somewhat simplified by Gernez/ who found that by bringing into contact with a supersaturated solution of para-tartrate of sodium and i Jungfleisch : Bull. Soc. CHm. [2] 19, 194. Comptes Rend. 76, 286 » Pasteur (Comptes Rend. 81, 128) rejects the instance unconditionally . J that no to the present time no active substance has been derived from inactive sub stances, and that, therefore, optical activity affords a definite distinguishing charac- teristic between natural and artificial substances 3 Pasteur: Ann. Chim. Phys. [3] 24; 442; 28, 56 ; 38, 4 Gemez : Liebig's Ann. 143, 376.](https://iiif.wellcomecollection.org/image/b28125952_0052.jp2/full/800%2C/0/default.jpg)