Handbook of the polariscope and its pracitcal applications / adapted from the German editon of H. Landolt, by D.C. Robb and V.H. Veley.
- Hans Heinrich Landolt
- Date:
- 1882
Licence: Public Domain Mark
Credit: Handbook of the polariscope and its pracitcal applications / adapted from the German editon of H. Landolt, by D.C. Robb and V.H. Veley. Source: Wellcome Collection.
70/320 (page 50)
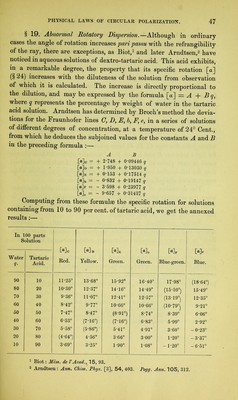
![By taking as unit-density that of water at 4° Cent., we make density synonymous with weight in grammes of 1 cubic centimetre of the substance, and the specific rotation of any active substance may then be defined as the deviation produced by 1 gramme of the substance when occupying a space of 1 cubic centimetre, and forming a column of 1 decimetre in length for the ray to traverse. The diameter of the column is thus immaterial. Moreover, since not only the density, but apart from that the rotatory power of an active liquid, is affected by changes of tempera- ture, the specific rotation will vary with the temperature, and it therefore becomes necessary to record the readings of the thermometer when a and d are observed. At any given temperature, the specific rotation of an active liquid in a state of purity is always constant. § 21. For active solid substances brought into the liquid state by solution in optically inactive and chemically indifferent solvents, the specific rotation is determined as follows Let P grammes of the active substance be dissolved in E grammes of inactive liquid, and d be the density of the resulting solution. Then the latter will contain in unit volume (cubic centimetre) grammes of active substance. If a solution of the above composition, in a tube l decimetres long, gives an angle of rotation a, then the deviation for a solution containing 1 gramme of active substance in 1 cubic centimetre of solution—i.e., the specific rotation [a]—is deducible from the proportion P P + M d ' l whence, [a] 1 : [a], a (P + E) l.E . d P If, with Biot, we indicate -p—- ^, substance in the unit weight of solution, by e, then [a] = that is, the amount of active / . e . d Lastly, if the proportion of active substance be stated for 100 parts by weight of solution, so as to make the numbers more con-](https://iiif.wellcomecollection.org/image/b28125952_0070.jp2/full/800%2C/0/default.jpg)