Handbook of the polariscope and its pracitcal applications / adapted from the German editon of H. Landolt, by D.C. Robb and V.H. Veley.
- Hans Heinrich Landolt
- Date:
- 1882
Licence: Public Domain Mark
Credit: Handbook of the polariscope and its pracitcal applications / adapted from the German editon of H. Landolt, by D.C. Robb and V.H. Veley. Source: Wellcome Collection.
85/320 (page 65)
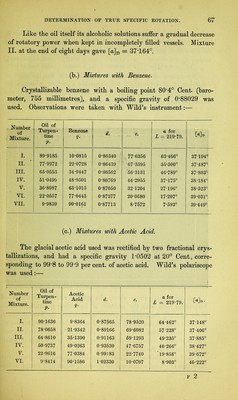
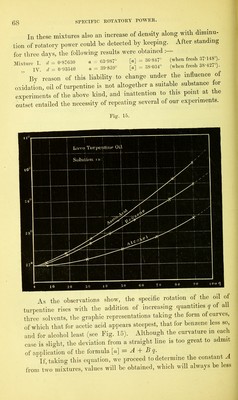
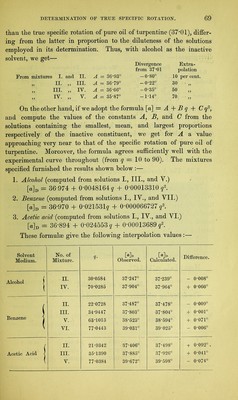
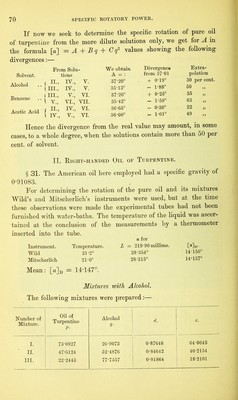
![admixture with different inactive liquids.1 In these, the value [a] exhibits very different rates of increase or decrease for increasing percentages of solvent q, the law of variation, when shown graphically, appearing in some cases as a straight line, in others as a curve. In the first place a formula was deduced for each, embracing all the curve lying between the most concentrated and the most dilute of the solu- tions used ; and, as the observations for the most part started with solutions containing about 90 per cent, of active substance, it was to be expected that at this point the value deduced for the constant A would approximate very closely to the true specific rotation of the absolute substance. Next it was sought to determine to what extent the calculated differs from the true specific rotation when only dilute solutions are used, as would be the case with substances but sparingly soluble. As already stated (§ 23), we find that when an active substance is dissolved in different liquids, different values of [a] are always ob- tained, even when the degrees of concentration are the same, whence it has been often inferred that for each solvent any substance has a different constant of specific rotation. Experiment in this direction must decide whether the true rotatory power of a substance does indeed suffer an immediate definite alteration under the influence of small proportions of inactive solvent, which subsequently proceeds at a different rate on further dilution, or whether the values obtained for constant A with different solvents do sufficiently agree with each other. Biot3 has recorded a solitary experiment of this kind, in which he worked with solutions of camphor in acetic acid and in alcohol. The results for the red ray (§ 18) gave the formulae— A B Solutions in acetic acid [a]r = 42 54 — 0T4236 q, alcohol [o]r = 45-25 - 0-13688 q. The two values for A, instead of agreeing as expected, here exhibit a by no means insignificant difference, and Biot assumes, in explanation, that the law of variation of the specific rotation is not expressed with sufficient exactness by the foregoing interpolation- formulae. The observations appended were made by the methods described at length in Chapter Y. 1 Liebig’s Ann., 189, 241. 2 Biot : Ann. Chim. Phys. [3], 36, 301, 307- F](https://iiif.wellcomecollection.org/image/b28125952_0085.jp2/full/800%2C/0/default.jpg)