Handbook of the polariscope and its pracitcal applications / adapted from the German editon of H. Landolt, by D.C. Robb and V.H. Veley.
- Hans Heinrich Landolt
- Date:
- 1882
Licence: Public Domain Mark
Credit: Handbook of the polariscope and its pracitcal applications / adapted from the German editon of H. Landolt, by D.C. Robb and V.H. Veley. Source: Wellcome Collection.
92/320 (page 72)
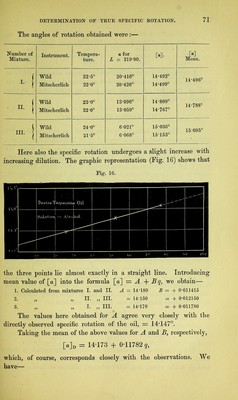
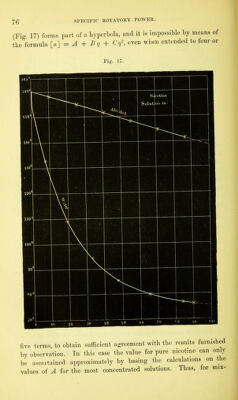
![Mixture. I. II. III. [«] Observed, 14-496 14- 788 15- 095 W Calculated. 14-490 14- 791 15- 089 Difference, - 0-006 + 0-003 - 0-006 A further series of experiments with benzene as solvent led to no result, as the oil of turpentine used in preparing the mixtures was not uniform, from not all having been kept an equal length of time exposed to oxidizing influences. III. Nicotine (L.zevo-rotatory). § 32. The pure substance here employed was prepared from 400 grammes commercial nicotine (suppliedby H. Trommsdorff, of Erfurt). To remove any small impurities of ether, alcohol, and water, the liquid was heated in a retort, whilst a stream of hydrogen passed over it, for eight hours, at a temperature of 150°, which was finally raised to 180° Cent., whereby a total distillate of 15 cubic centimetres, consisting chiefly of alcohol, passed over. The residue was then distilled in a current of hydrogen, in successive portions of 200 grammes, from a small retort heated by a sand-bath. At 225° Cent, the nicotine, at first still retaining some water, began to pass over, but the thermometer immersed m the liquid quickly rose to 244° (corrected, 249° Cent.) when the boiling proper began. Raised into the vapour, the thermometer fell to 241*5° to 242° (corrected, 246*6° to 246*8° Cent.), which temperature re- mained constant during the rest of the distillation. Height of baro- meter, 745 millimetres. The hydrogen was admitted m a very slow stream, and no decomposition of the substance resulted. Altogether 350 grammes of pure substance, in the form of a colourless, liquid, faintly tinged with yellow, were obtained. It was sealed up in glass tubes. An analysis of this nicotine, the nitrogen being determined in the gaseous form, gave the following numbers . Required for Found. c10 h14 n2. c 74-02 73-95 H 8-66 8-92 N 17-32 17-55 The preparation was submitted to further examination by titra- tion. The nicotine was weighed in thin glass bulbs, which were then broken under water, and, after solution, tincture of litmus added,](https://iiif.wellcomecollection.org/image/b28125952_0092.jp2/full/800%2C/0/default.jpg)