Volume 1
Allen's commercial organic analysis : a treatise on the properties, modes of analysis, and proximate analytical examination of the various organica chemicals and products employed in the arts, manufactues, medicine, etc. with concise methods for the detection and estimation of their impurities, adulterations, and products of decomposition.
- Alfred Henry Allen
- Date:
- 1911-1917
Licence: Public Domain Mark
Credit: Allen's commercial organic analysis : a treatise on the properties, modes of analysis, and proximate analytical examination of the various organica chemicals and products employed in the arts, manufactues, medicine, etc. with concise methods for the detection and estimation of their impurities, adulterations, and products of decomposition. Source: Wellcome Collection.
61/604 (page 45)
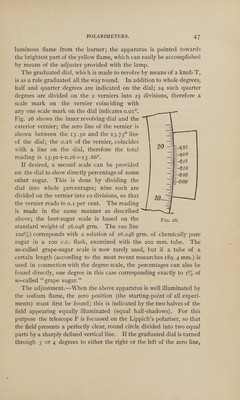
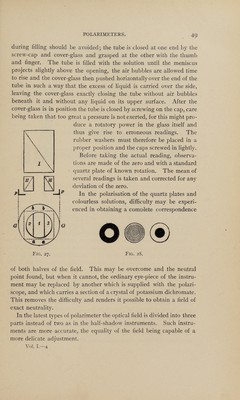
![spectrum of the particular ray employed. In practice the rotation of a substance is expressed in two ways. Either it is referred to the D line of the solar spectrum, the rotation being then expressed by [a]D; or it is referred to the “medium yellow ray” (jaune moyen) which is com¬ plementary to Biot’s transition tint; the rotation being then denoted by [a]j. In the former case [a]D is measured by a Wild, Mitscherlich, Jellet-Cornu or Laurent instrument and the direct rotation is found in degrees of arc. In the latter case, [a] • is measured by means of a neutral-tint or half-shadow polarimeter, such as the Ventzke-Scheibler. The scale divisions in such instruments are arbitrary and have to be con¬ verted into angular degrees before the specific rotatory power can be calculated. The readings obtained being based on the rotation of a quartz plate are obtained in terms of the rotation of a quartz plate of definite thickness. Whilst the rotatory dispersion of quartz and cane- sugar solutions are nearly identical, most other substances have a very different rotatory dispersion from that of quartz. Thus, a quartz com¬ pensating instrument can only be used in the comparison of rotatory powers of different substances, when the rotatory dispersion of the substance under examination is known relatively to that of quartz. The wave length of the “mean yellow” ray being less than that of the D line, the numerical value of [a] • is greater than that of [a]D. With a quartz plate i mm. thick, Broch found Md= 21.67 [a],'=24 ■ 5 Whence: [«],•= 1.1306 [a]D or approximately 9/8 [a]D. [a]D = 0.8845 [a]j or approximately 8/9 [a]r he proportion between [a] • and [o]D varies in different substances owing to their having a different rotatory dispersion. For sugar solutions [u]y : [a]D = 1.129. (Very nearly the same as for quartz.) For camphor solutions in alcohol the ratio is 1.198 and for oil of tur¬ pentine 1.243. Use of the Polarimeter.—Fig. 25 shows a Schmidt & Haensch polarimeter of the Lippich half-shadow type with divided circle reading by magnification to o.oi°. It is intended for use with a sodium flame, a gas burner for this purpose being supplied with the instrument. This instrument is chosen as being a typical half-shadow](https://iiif.wellcomecollection.org/image/b31360798_0001_0061.jp2/full/800%2C/0/default.jpg)