A text-book of pharmacology, therapeutics and materia medica / by T. Lauder Brunton ; adapted to the United States Pharmacopoeia by Francis H. Williams.
- Lauder Brunton
- Date:
- 1891
Licence: Public Domain Mark
Credit: A text-book of pharmacology, therapeutics and materia medica / by T. Lauder Brunton ; adapted to the United States Pharmacopoeia by Francis H. Williams. Source: Wellcome Collection.
73/1324 (page 17)
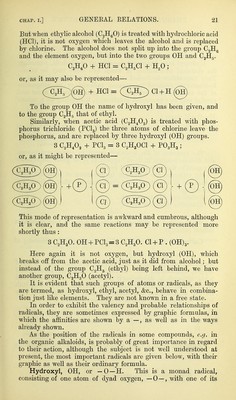
![The first important attempt at a natural classification of the elements was made by Newlands in 1864.^ He then arranged them in g-roups, be- tween the members of which there was a close connection in regard to their chemical properties, and a curious relation in regard to their atomic weights. These presented differences which were generally multiples of the atomic weight of hydrogen, and generally equal to, or multiples of, that of oxygen. A curious relationship had also been pointed out by M. Dumas between the members of the potassium group, their atomic weights being equal to multi]Dles of those of lithium and potassium added together. Li + K = 2Na, or in figures, 7 + 39 = 46 Li + 2K = Eb „ 7 + 78 = 85 2Li + 3K = Cs (133)3 „ 14 + 117 = 131 Li + 5K = Tl (203-7) „ 7 + 195 = 202 3Li + 5K = 2Ao- „ 21 + 195 = 216 A similar relation was also pointed out by Mr. Newlands between lithium and the calcium group ; as follows :— Li + Ca = 2Mg (48), or in figures, 7 + 40 = 47 Li + 2Ca = Sr „ 7 + 80 = 87 2Li + 3Ca = Ba (137) „ 14 + 120 = 134 Li + 5Ca Pb 7 + 200 - 207 But Mr. Newland's most important table is the following one, in which he has arranged the elements m ten series :— Triad Lowest term Mean Highest term 1. Li 7 + 17 =Mg24 Zn 65 Cd 111-8 II. B 11 III. C 12 + 16 =Si 28 Sn 118 IV. N 14 + 17 =P 31 As 75 Sb 120 V. 0 16 + 16 =S 32 Se 78-8 Te 128 VI. F 19 + 16-5 = CI 35-5 Br 80 I 127 VII. Li 7 -t-16 = Na 23 + 16 =K 39 Kb 85-3 Cs 133 VIII. Li 7 + 17 = Mg 24 + 16 -Ca40 Sr 87-4 Ba 137 IX. V 51-3 W 184 X. Mo 95-5 Pt 195 Pd 105-7 Au 196 + 88 = Bi 210 + 70-Os 199 + 70 = T1 203 + 70 = Pb 207 Seven of these series nearly correspond in their first members with those of Mendelejeff, to whom and to Lothar Meyer we owe the complete develop- ment of this mode of classification. Mr. Newlands also pointed out that the eighth element starting from a given one, was a kind of repetition of the first, like the eighth note of an octave in music.^ Mendelejeff has not only greatly developed this system of classification, but has afforded convincing proof of its value by not only predicting the existence of an unknown element, but actually describing its physical cha- racters and chemical reactions—a prediction the correctness of which was proved by the discovery of gallium, and by the agreement of its characters and reactions with those which Mendelejeff had foretold. The various members of the animal kingdom can all be arranged in a few series : Protozoa, Coelenterata, Annuloida, Annulosa, Molluscoida, Mollusca, and Vertebrata. These series all differ more or less from one another, but a ' Newlands, Chemical Neios, July 30, 1864. - Dumas, quoted by Newlands, op. cit. ^ The newer atomic weights of Cs, Fl, Mg, and Ba do not correspond so exactly as their old ones with the sum of the other elements. Chem. News, Aug. 20, 1864, p. 94. C](https://iiif.wellcomecollection.org/image/b20410001_0073.jp2/full/800%2C/0/default.jpg)