Handbook of materia medica, pharmacy, and therapeutics : including the physiological action of drugs, the special therapeutics of disease, official and practical pharmacy, and minute directions for prescription writing / by Sam'l O.L. Potter.
- Samuel Otway Lewis Potter
- Date:
- 1897
Licence: Public Domain Mark
Credit: Handbook of materia medica, pharmacy, and therapeutics : including the physiological action of drugs, the special therapeutics of disease, official and practical pharmacy, and minute directions for prescription writing / by Sam'l O.L. Potter. Source: Wellcome Collection.
Provider: This material has been provided by Royal College of Physicians, London. The original may be consulted at Royal College of Physicians, London.
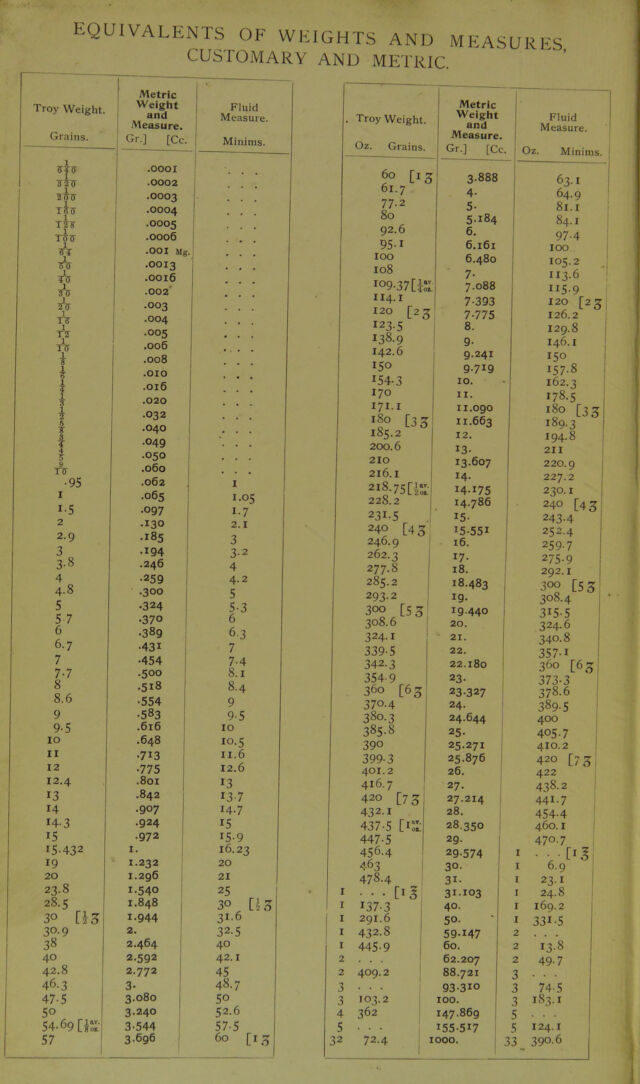
27/944 page 21
![separated by water. The active principles are alkaloids, acids, salts, glucosides and other neutral principles, volatile oils, resins, etc. Some few of these may be extracted by Water alone (e.g., Morphine), and in some cases the addition of acids or alkalies to the water will effect the chemical solution of many ingredients which are insoluble in water alone. As a rule, however. Alcohol is the most generally applicable of all simple solvents, but from its hardening the cell-membranes instead of softening them it prevents the osmosis of their contents. Drugs subjected to alco- holic or ethereal menstrua should have their cells thoroughly broken or torn, so that the solvent may be brought into actual contact with the principles contained in them. The degree of disintegration required depends upon the size of the cells, ducts, tubes, intercellular spaces, etc., in which the active principles are enclosed. A very finely powdered state is however open to objection from the packing of the particles together into an almost impenetrable mass when treated by the solvent. The average size of vegetable cells is about of an inch, while resin cells and other cavities are larger, averaging perhaps about inch. The Pharmacopoeia prescribes in each instance the degree of fineness of the powdered drug employed in making certain of its preparations, or its bruising, slicing, etc., when such operations will answer. [Compare the article on Comminution in Part II.] Alkaloids are active, nitrogenous principles existing in plants, from which they are extracted by chemical art. They are organic bases, com- bining with acids to form crystalline salts without the production of water. They are regarded as compound ammonias, that is to say, one or more atoms of H in ammonia (NHj) are in them replaced by various radicals; and they may be considered to be products of albuminous decomposition in the plant-cells during the process of growth. Like ammonia, they all contain N, with C and H; most of them also contain O, though a few are devoid of the latter element, and occur as oily liquids, namely—Nico- tine, Coniine, Sparteine, Piperidine, Lupuline, Lobeline, Muscarine and Pilocarpine. Alkaloids are alkaline in reaction, the solid ones (except Berberine) are colorless; they are sparingly soluble or insoluble in water, but readily soluble in alcohol; while their salts are more soluble in water than in any other liquid. Their solutions are intensely bitter. They are easily decomposed by alkalies or alkaline carbonates ; and are precipitated from their solutions by a solution of Iodine in Potassium Iodide, by Po- tassio-mercuric Iodide, and by Picric, Phospho-molybdic and Phospho- tungstic Acids. They generally have a powerful physiological action. They are closely related to Pyndene, and some of them may be synthet- ically prepared from pyridene bases. The names of alkaloids terminate in Latin in —ina, in English in —ine.](https://iiif.wellcomecollection.org/image/b24907303_0027.jp2/full/800%2C/0/default.jpg)