On food : four Cantor lectures, delivered before the Society for the Encouragement of Arts, Manufactures, and Commerce / by H. Letheby.
- Henry Letheby
- Date:
- 1868
Licence: Public Domain Mark
Credit: On food : four Cantor lectures, delivered before the Society for the Encouragement of Arts, Manufactures, and Commerce / by H. Letheby. Source: Wellcome Collection.
Provider: This material has been provided by The Royal College of Surgeons of England. The original may be consulted at The Royal College of Surgeons of England.
22/56 (page 20)
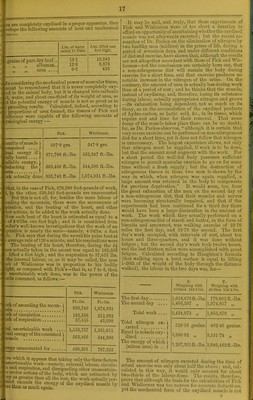
![mentaiy scales, ogg-shoUs, &c., of the vertobratn. In all llicsu instances tho secreted matter must first have been crystalloidal, or it could not have been secreted; it then lakes tho form of a liquid colloid or jolly ; and finally, by a further molecular movement, it passes into tho condi- tion of a pcctous solid—tho saline constituents, accord- ing to their naturo and ijroportion, determining tho degrees of hardness. Agiiin, tho removal of efifeto matters and worn-out tissues is undoubtedly effected by tho agency of saline substances; for, during the processes of oxydation, acid compounds are produced, which, by acting chemically on the saline constituents of the animal fluids, givo them a solutivo power on plastic matters, and thus enable them to remove tho debris of worn-out tissue. As to special functions of the several saline constituents of foo 1, little can bo said ; but it is a remarkable fact ihrit th.0 a/ka/ine or basic phosphate of soda is invariably found in tho blood, while acid phosphate of potash is the chief constituent of the juice of flesh. Most likely the former is concerned in preserving the liquid colloidal condition of albumen and fibrin, and so keeping them from being lost by secretion, whilo the latter is engaged in an opposite duty. The alkalinity of the blood also helps in the oxydation of organic matters; and as the basic phosphate of soda is endowed, like an alkaline car- bonate, with the power of absorbing carbonic acid, it is the chief agent whereby this compound is removed from the system. This is a remarkable property, and is one of the chief uses of basic phosphate of soda in the blood. In point of fact, when it is not there in sufiS.cient quantity to perform this function, it is replaced by an alkaline carbonate. We find this to be so in the blood of herbivorous animals, where the proportions of the two salts are the reverse of what they are in man and car- nivora. Some notion may be formed of the relative importance of the saline matters of the blood by refer- ence to this diagram from Liebig. Percextage Composition of the Mineeal Matters OF Blood. Man. Pig-. Dog. Fowl. Sheep. Ox. Mineral Acidsand O.Kido 1 31-79 05-66 3-33 9-22 36 50 49-80 3-80 9-90 36-82 55-24 2-07 6-87 47-26 43-41 2-22 2-11 14-80 55-79 4-87 24-54 14-04 60 00 3-64 22-32 100-00 100-00 100-00 100-00 100-00 100-00 And in those cases where the phosphoric acid is deficient, ib is replaced by carbonic acid. In man, for example, the quantity of combined carbonic acid in the ashes of the blood is only 3-78 per cent., whereas in tho calf it is 0-8;j, and in tho sheep 19-47 per cent., so that in all casos the alkalinity of the blood remains the same. Tho salts ofiMtash in tho juice of flesh have, no doubt, an equally important duty to perform, although of an opposite character; for whilo the alkaline phosphate of ijd'i in the blood prevents the transudation of nutrient matter, tho acid phosphate of potash in the muscular fluid promotes it; and thus it is concerned in nutrition and in tho solution of worn-out tissues. Enrlhij phosphates, especially of lime, aro, ])orhrip3, the agents for tho consolidation of tissue; for not only arc they present in the hnrd structures of the body, as tho bones and teeth, but they also enter into the composition of flesh. And not less impoitant in tho morphological functions (f the animal body is the presenco of commnn salt. It is a largo cohstituciit of every one of tho secretions, and forms about half tho total weight of the saline matters of Iho blood. Unlike tho phosphates, however, it docs not ( liter into tho composition of tissue, but seems to be only I mclium of absorption and secretion ; and so necessary i i it for this purpose, that it is not possible to alter, to any largo extent, its proportion in tho blood. If vro drink water containing but little common salt in solutior it does not permanently dilute the blood, but pa- imraediately by the kidneys; and if wo try to in the amount in the blood by drinliing solutions of sail,. sea water, it refuses to be absorbed. This normal pr- portion of it in the blood is evidently a physio! necessity, which tho conditions for diffusion imper,. demand. It is a curious fact, also, that common sail iamA-: tho faculty of foi-ming crystallizable compounds -wHRf*' most of the unorganised and cfi'ete constituents of tl body. INIay it not, therefore, be an impoi'tant agent difl'usion, and bo thus concerned in the phenon: absorption and secretion; for as colloidal matters- men and fibrin—cannot pass through the walls of ti intestines, or tho blood-vessels, it may well bo th:. through the agency of common salt and tho free acid of tL gastric and muscular juices, they temporarily assumej crystalloidal condition, and arc thus absorbed or secreti The constant presence of common salt in the secretiq and the necessifry for it in due proportion in the bla indicate the importance of a proper supply of it with i food. Wo perceive this in the instinct of animals, in our own cra-ving for it when it does not exist in su cient quantity in the food. Animals, in fact, will 1 long distances, and brave the greatest dangers, to obti it. Men will barter gold for it; indeed, among Gallas, and on the coast of Sierra Leone, brothers -wiT' sell their sisters, husbands their wives, and parents tht children, for salt. In the disti-ict of Accra, on the Go. Coast of Africa, a handful of salt is the most valual. thing upon earth after gold, and -svill purchase a slavi * or two. Mungo Park tells us that with the Mandingoei-#> and Bambaras the use of salt is such a luxury, that t say of a man he flavours his food -with salt is to imp. that he is rich ; and children vnH suck a piece of roc'i salt as if it were sugar. The experiments of Boussingault have shown that although salt mixed with the fodder of animals does no much affect the quantity of flesh, fat, or milk obtain- from them, yet it seriously affects theii- appearance an general condition; for animals deprived of salt, othajp: than that contained naturally in the food, soon get heav: andduUin their temperament, and have _ a rough ar staring coat. Eeulin states that animals which do not fir it in their food or drink, become less prolific, and ti breed rapidly diminishes in nrunber. This is confirm by Dr. Lo Saine, who saj-s, in his prize-essay on sa. that it increases the fertility of the male and tL fecundity of tho female, and it doubles the power 0^ nourishing the foetus. During the period of sucklinn also, salt given to the mother renders the milk mf ; abundant and more nutritious. It likewiso acci' growth, and gives a finer condition to the skin; : flesh of animals fed with it is better flavoured, ana ii. easily digested, than that of animals which do not parti, of it. In barbarous times, the most horrible of pum^ ments, entailing certain death, was the feeding of culpr. on food without salt; and in the experiments of i- French Academicians, flesh deprived of its saline cc: stituents by being washed with water, lost its nutnbv . power, and animals fed on it soon died of starvaUoij Even after a few days, with such a diet, the instincts c | the animals told them it was worthless as food; indeecj for all purposes of nutrition, it was, as Liebig says, nj better than the eating of stones, and the utmost tormont- of hunger wore hardly sufficient to induce them to cc- tinuQ the diet. There was plenty of nitrogenous matt, in the food, but there was no medium for its soluUa^c; and absorption, and hence it was useless. The oxides of iron, and their homologues, the oxide-' iiiaiiiffiiiese, are largely concerned in the processes i •= ' o-uification and oxydation. They enter into the sition of tho globules of the blood—manganese be i. chi-f mineral constituent of the corpuscles of wni> blooded aTiinial.s, and iron of red. In fact, the colounr.- matter of the blood discs (cruorin), as well as that on muscles (myochi-omc), ia a compound of iron ana aiw](https://iiif.wellcomecollection.org/image/b22280364_0022.jp2/full/800%2C/0/default.jpg)