Qualitative chemical analysis and laboratory practice / by T.E. Thorpe and M.M. Pattison Muir.
- Thomas Edward Thorpe
- Date:
- 1894
Licence: Public Domain Mark
Credit: Qualitative chemical analysis and laboratory practice / by T.E. Thorpe and M.M. Pattison Muir. Source: Wellcome Collection.
28/273 (page 6)
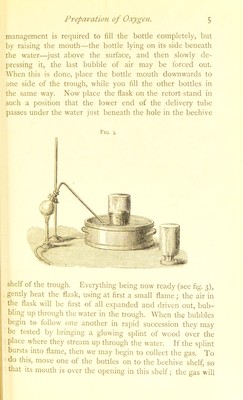
![then rise into the bottle, displacing the water, which finds its way into tlie trough. When full, depress the mouth of the bottle somewhat further beneath the water, slip a small tray under it, then lift the bottle, standing mouth downwards, on the tray (which must contain a little water) out of the trougli, ami set it aside. bill the other three bottles in a similar manner. In heating the flask while preparing the gas, so regulate the heat as to avoid any sudden rush of gas ; and whenever a sufficient amount of o.xygen has been collected, remove the lamp and lift the end of the delivery tube out of the water, otherwise, as the flask cools, the water will rush back into it and crack it. The mass remaining in the flask may, when cold, be easily washed out with water. We will now proceed to examine the properties of the o.xygen we have obtained. By the preliminary experiment with ])otassium chlorate heated in the test-tube we have learned that a burning body when plunged into oxygen burns with greatly increased activity. Oxygen is evidently a supporter of combustion. Experiment 1.—Take a small piece of amorphous phos- phorus about the size of a pea, and place it in the cup of a deflagrating spoon, set fire to the phosphorus by bringing it into a gas flame, and plunge it into a bottle of oxygen : a brilliant white light, almost insuppoitably bright, is produced, together with dense white fumes. (If the gas bottle be made of thick glass, it is advisable to dilute the oxygen in it with about one-third of its volume of air before burning the phosphorus, as the great heat may crack the bottle. This may be done by depressing the bottle in the trough, allowing one-third of the oxygen to escape, and then lifiing the bottle, mouth downwards, out of the trough, so that the water which flowed in when the oxygen escaped, may run out.) When the bottle rs cool, remove the deflagrating spoon, put a little water into'the bottle, and shake it up; the white fumes drs-](https://iiif.wellcomecollection.org/image/b28122768_0030.jp2/full/800%2C/0/default.jpg)