Qualitative chemical analysis and laboratory practice / by T.E. Thorpe and M.M. Pattison Muir.
- Thomas Edward Thorpe
- Date:
- 1894
Licence: Public Domain Mark
Credit: Qualitative chemical analysis and laboratory practice / by T.E. Thorpe and M.M. Pattison Muir. Source: Wellcome Collection.
42/273 (page 20)
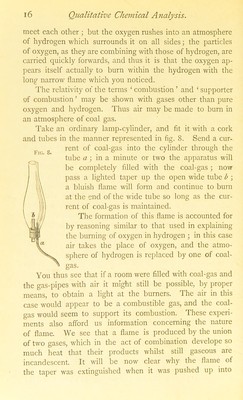
![KNO 3 + HjSO, = HNO3 + KHSO,—that is to say, 39 + 14 + 48 = loi parts by weight of potassium nitrate are decomposed by— ' 2 + 32 + 64 = 98 parts by weight of sulphuric acid ; one of the products being— I + 14 +48 = 63 parts by weight of nitric acid. Calculate then the amount of strong sulphuric acid re- quired to decompose the 30 grams of nitre, weigh out this amount in a small beaker, and pour it into the retort through a small funnel passing well into the body of the retort through the opening at the neck. Put in the stopper and su])])ort the I'etort, with wire gauze beneath it, on one ring of a retort-stand, bringing another ring over the neck. Clean and dry a small flask, the neck of which will permit the Kic. 10. I)eak of the retort to be passed through it; support it on the beehive shelf, and fill the trough witli water, so that the flask may be pretty well covered, but take care that no water gets into it. I’he apparatus has now the appearance shown in Fig. 11. Gently heat the retort : an action evidently goes forward ; after a little time brown fumes are evolved, and drops of liquid form on the sides of the neck, and trickle slowly down it into the small flask, or receiver, which being kept cool by the water surrounding it, condenses the nitric acid, which in the heated retort is in a gaseous state. In the receiver a brownish-yellow heavy liquid, fuming](https://iiif.wellcomecollection.org/image/b28122768_0044.jp2/full/800%2C/0/default.jpg)