The commercial hand-book of chemical analysis : or, practical instructions for the determination of the intrinsic or commercial value of substances used in manufactures, in trades, and in the arts / [A. Normandy].
- Alphonse Le Mire de Normandy
- Date:
- 1875
Licence: Public Domain Mark
Credit: The commercial hand-book of chemical analysis : or, practical instructions for the determination of the intrinsic or commercial value of substances used in manufactures, in trades, and in the arts / [A. Normandy]. Source: Wellcome Collection.
30/550 (page 10)
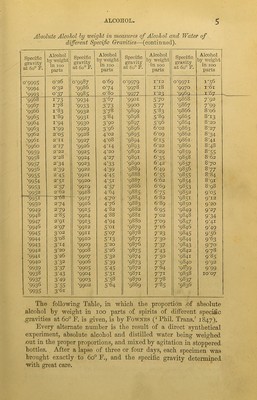
![Fig. 4. ID 20 30 60 70 80 90 thumb, so as to allow the acid to trickle iu drops as occasion may require ; and it is well also to smear the lip of the tube with tallow, in order to prevent any portion of the test-acid from being wasted by running over the outside, after pouring, which accident would, of course, render the analysis altogether inaccurate and worthless ; and for the same reason, after having once begun to pour the acid from the alkalimeter, by allowing it to trickle between the thumb and the lip of the tube, as above mentioned the thumb must not be removed from the tube till the end of the experiment, for otherwise the portion of acid which adheres to it 40 would, of course, be wasted, and vitiate the result. 5Q With either of the alkalimeters, Figs. 2 and 3, this precaution is not required, the acid falling naturally, drop by drop, from the small tube, by inclining the alkalimeter. There are several other forms of burette or alkali- meter. Fig. 5 is the form known as Bfstks’s burette. The drop tube is here at the top instead of at the bottom of the tube. The use of this instrument re- quires a steady hand, but after a little practice a liquid may be delivered from it with great precision. Mohr’s burette is shown in Fig. 6. It consists of a graduated tube drawn out at the end, on which is slipped a small piece of india-rubber tubing, in the end of which is inserted a very small glass tube as a mouthpiece. The india-rubber is confined by a brass spring or pinch-cock, shown in Fig. 7. ‘ The advantages possessed by this instrument,’ observes Mr. Sutton (‘Handbook of Volumetric Analysis’), are that its constant upright position enables the operator at once to read off the number of degrees of test solution used for any analyses. The quantity of fluid delivered can be regulated to the greatest nicety by the pressure of the thumb and] finger on the spring clip, or pinch-cock, and the instrument not being held in the hand, there is no chance of increasing the bulk of the fluid by the heat of the body, and thus leading to incorrect measurements, as is the case with Binks’s or Gay-Lussac’s form of instrument. Preparation of the Standard Acid (modification of Gay- Lussac’s method).—Ordinary oil of vitriol is diluted with ten or twelve parts by measure of water, and allowed to cool. A quantity of the best bicarbonate of sodium is washed on a filter with cold water till the filtrate, when neutralized with pure nitric acid, ceases to give out a precipitate with either nitrate of silver or](https://iiif.wellcomecollection.org/image/b28078172_0030.jp2/full/800%2C/0/default.jpg)