A manual of chemistry : theoretical and practical, inorganic and organic, adapted to the requirements of students of medicine / by Arthur P. Luff and Hugh C.H. Candy.
- Arthur P. Luff
- Date:
- 1910
Licence: In copyright
Credit: A manual of chemistry : theoretical and practical, inorganic and organic, adapted to the requirements of students of medicine / by Arthur P. Luff and Hugh C.H. Candy. Source: Wellcome Collection.
Provider: This material has been provided by Royal College of Physicians, London. The original may be consulted at Royal College of Physicians, London.
39/644 (page 23)
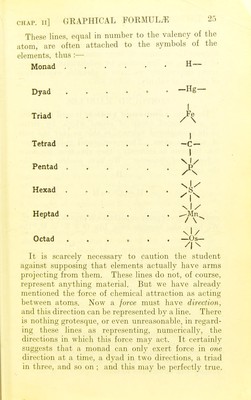
![CHAP. Il] hydrogen atoms. As. the hydrogen atom seems to possess unit valency and to correspond to the penny coin in our chemical currency, it is called a monad or monovalent atom. The aluminium atom is there- fore a triad or trivalent one. It seems possible for valency to have any integral value from 1 to 8. Variation in valency.—It is, of course, possible with a sixpenny coin to employ only a part of its purchasing power and to hold the remainder in re- serve. In this respect also the valency of an atom corresponds to the purchasing power of a coin. A pentavalent atom, such as the nitrogen atom, may, for instance, use only a part of its full valency and hold the remainder in reserve in some way. We must, however, regard the maximum valency of an atom as its true valency, and account for its exercise of lower valencies in this way; when an atom is exerting its maximum valency, the compound it forms is said to be saturated. Such compounds appear to be more stable than unsaturated ones. The valency of an atom may be measured, not only by the number of hydrogen atoms for which it can be exchanged, but also by the number of hydrogen atoms with which it can combine. Instances of a higher valency than eight are unknown, and the polyvalent elements seem only to exert their maxi- mum valency on rare occasions. Thus phosphorus forms PH3 in preference, apparently, to PH5, and sulphur SH2 in preference to SH6. S03 is, however, a well known compound, and since oxygen never seems to exert a lower valency than 2, we argue that sulphur has here a valency of 6. Similarly, from P205 we argue that phosphorus is a pentad. Facts like these teach us that there is something more than a mere numerical difference between valency towards one element and valency towards another. They suggest that valency is really a dual or re-](https://iiif.wellcomecollection.org/image/b22651603_0039.jp2/full/800%2C/0/default.jpg)