Tables of physical and chemical constants and some mathematical functions / by G.W.C. Kaye and T.H. Laby.
- G. W. C. Kaye
- Date:
- [1941]
Licence: Public Domain Mark
Credit: Tables of physical and chemical constants and some mathematical functions / by G.W.C. Kaye and T.H. Laby. Source: Wellcome Collection.
143/200 (page 131)
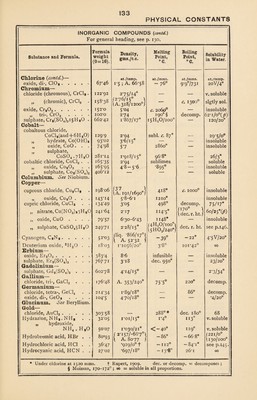
![PHYSICAL CONSTANTS INORGANIC COMPOUNDS (contd.) For general heading, see p. 130. Substance and Formula. Formula Density, Melting Boiling Solubility weight gms./c.c. Point, Point, in Water. ;0 = 16). °C. °C. Ammonium (contd,.)— at./temp. at./mms. at./mms. at./temp. nitrite, NH4N02 .... oxalate, (NH4)2C204. HaO. 64*05 1*69 decomp. — soluble 142*1 1*502 — — 4/i5° persulphate, (NH4)aSa08 . phosphomolybdate, 228*2 decomp. 1 58/0° *03/15° (NH4)sP04 . i2Mo03.3H20 193-1 — — •— sulphate, (NH4)2S04 . . . 132*14 1*77/20° 140° f dec. 250° J 76/20° sulphocyanate, NH4CNS . Antimony— 76*12 1*306/13° 159° dec. 170° 162/20° bromide, SbBrs. 360*0 4*15/23° 94*2° 280° decomp. chloride, tri-, SbCl3 . . . 226*6 306/26° A. 234 73* 20 223° 1816/150 \ CO jj 2° „ penta-, SbCl5 . . 297*5 2*35/20° 2*8° io2°/68 decomp. hydride, SbH3. 123*2 A. 124*5/15° - 91*5° —18° 20 V. iodide, tri-, Sbl3 .... 501*0 14*85/26° \A. 509*5 170*8° \ subl. 114°/ 401° decomp. oxide, tri-, Sb203 .... 288*4 5*2-57 red heat 1550° *002/15° „ tetr-, Sba<J4 .... 304*4 4*0 7 0/8oo° insoluble „ pent-, Sba06 . . . potassium tartrate, 320*4 3*8 0/300° Oa/8oo° insoluble S5/9* . I36/IOO K(Sb0)C4H404-*Ha0 332*36 2*6 £H20/ioo° decomp. sulphide tri-, Sb2S3 . . . 336*6 4-65 546° volatilizes insoluble „ penta-, SbaS3 . . 400*7 4*12/0° fusible — insoluble Arsenic— bromide, AsBr3 ..... 314*7 / 3-66/15* ] \A. 315-8 / 3i° 221° decomp. chloride, AsC13. 181-3 2*17/0°; A. 182 - 18° I30*2° decomp. fluoride, tri-, AsF3 .... 132*0 2*7; A. 132 -8*5° 63° decomp. „ penta-, AsF6 . . . 170*0 — - 80° ~53° soluble hydride, AsH3. 77*98 A. 78 ~ii3*5° -54*8° slgtly sol. iodide, di-, Asl2. 328-8 — — „ tri-, Asl3 .... 455*7 4*4/13° A. 482 140*7 394-414° 30/100° „ pent-, AsI6 . . . . 709*6 3*93 70° decomp. oxide, tri-, As203 .... 197*9 3*86/25° A. 413 subl. 218° — 1*7/16° „ pent-, Ass03 . . . 229*9 3*9-4*2 red heat decomp. 245/12° Barium— bromide, BaBr2.2HaO . . 333*2 3*85/24° anhy. 88o° 2H*0/lOO° 103/15° carbonate, BaC03 .... 197*4 4*3 1360° * diss. 1450° •0022/18° chloride, BaCl,. 2H20 . . 244*3 3*1/24° anhy. 960° see p. 146. hydride, BaH2. 139*4 4*2/o° 1200° 1400° decomp. iodide, Bal2. 391*2 5*150/25° 740° — 170/0° nitrate, Ba(N03)2 . . . . 261*4 3*24/23° 575° — 5/o° oxide, BaO. 153*4 47 - 5'5 BaOa/45o° — 1-5/0° „ per-, BaO s . . . . 169*4 4*96 BaO/4500 — insoluble sulphate, BaS04 .... Beryllium — 233*4 4*476, 4*33 1580° ■1 *o323/i8° bromide, BeBra . . . . 168*9 — 6oi° subl. soluble chloride, BeCl3. sulphate, BeS04.4H20 . . 80*02 — 400° — v. soluble 177*2 1*7/10° dec. r. ht. 2H20/lOO° 44/30° i anhy. = anhydrous ; dec. or decomp. = = decomposes; r. ht. = red heat; subl.= sublimes ; v. = very ; 00 = = soluble in all proportions. * basic 95° 0. t M.P. of NH4HS04. J dec. without melting into NH4HS04.](https://iiif.wellcomecollection.org/image/b31356904_0143.jp2/full/800%2C/0/default.jpg)