Tables of physical and chemical constants and some mathematical functions / by G.W.C. Kaye and T.H. Laby.
- G. W. C. Kaye
- Date:
- [1941]
Licence: Public Domain Mark
Credit: Tables of physical and chemical constants and some mathematical functions / by G.W.C. Kaye and T.H. Laby. Source: Wellcome Collection.
153/200 (page 141)
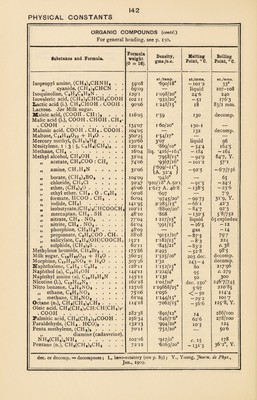
![PHYSICAL CONSTANTS ORGANIC COMPOUNDS {contd.) . —.. For general heading, see p. 130. Formula Density, gms./c.c. Melting Point, 0 C. Boiling Point, 0 C. Substance and Formula. weight (0 =s 16). at./temp. at./mms. at./mms. Ethyl propionate, C2H6C02C2H6 . . 102’11 *8901/20° -74*25 99*0° „ salicylate, C6H4(H0)C02. C2H5 l66'I3 1-138/15° 1*3 231-5 „ sulphide, (C2H5)2S. 90’l6 *837/20o — 99*5 92*6 tartrate (d.), C4H406(C2H6)2 . . 206*15 I*206/20g 17 280 „ valeriate, QHgCOj^Hf, . . . I30'I5 *876/20° 144*5 Ethylene, CH2 : CHa.. 28*04 C565/-102*5° 1A. 28*32 } -169 —102*7 „ bromide, di-, CH2Br . CH2Br 187*88 2*1838/18° 9*97 131*6 „ chloride, di-, CH2C1 . CH2C1 98*90 1*28/0° -35*3 83*7 ,, oxide, <C(CH2)20 .... 44'°4 •897/0° — in i3*5/746 Ethylidene chloride, CH3 . CHC12 . . 98*96 1*186/12° -967 59*9 Eucalyptol, Cl0H18O. 154*19 •927/20° — 2 176 Eugenol, C6H3 . (OH) . OCHs . C3H6 164*15 1*0620/25® liquid 247*5 Fluor benzene, C6H5F. 96*07 1*024/20° —41*2 85*2, Y. Formic acid, H . CO OH. Formaldehyde, H . COH. 46*02 1*218/20° 8*35° 100*5 30*02 •815/ — 2o°A.48 -92 -21 Fructose (d.), CH2OH[CHOH]3CO- CH2OH .. 180*13 1-55/0° 104 — Fumaric acid, (COOH . CH :)2 . . . 116*05 1*625 286 290 Furfural, C4HsO . COH. 96*06 1*159/20° -36*5 161 Galactose (d.), CHO[CHOH]4CH2OH 180*13 — 170 — Glucose (d.), CHO[CHOH]4CH2OH 4- H20.. 198*14 1*54-1*57 146 — Glutaric acid, COOH(CH2)3COOH 132*09 — 97*5 303 Glycerine, OHCH2. CHOH . CH2OH 92*08 1*26/20° 17 290 Glycocoll, glycine, CH,NH,COOH . 75’°7 1*161 c. 234 — Glycol, CH,OH . CH2OH .... 62*06 1*125/25° —17*4 197*4 Glycollic acid, CH2OH . COOH . . 76*04 — 78 decomp. Glyoxal, CHO . CHO. 58*03 1*14/20° ■5° 50*5° Glyoxalic acid, CHO . COOH + HaO . 92*04 syrup with steam Grape sugar. See Glucose. Heptane (n.), CH3(CH2)5CH3 . . . 10016 •6836/20° — 90*0 98*4, Y. Hexane (n.), CH3(CH2)4CH3 . . . 86*14 '6595/2°° -94*3 69, Y. „ di-isopropyl, [(CII3)2CH]2 86*14 *6617/20° -135 58*1, Y. 26*1 Hydrocyanic acid, HCN. 27*02 •697/18° -14 Indigo, C„H,<CO >C : C<£°>C6- H4. 262*18 1*35 390 — 2 subl. 156° Indol, C6H4NHCH : CH . . . . . 117*11 — 52 253-4 Iodoform, CHI3. 39377 4*08/17° 119 sub). & dec. Isatine, C6H4<^>COH .... 147*09 — 201 sublimes Isoamyl acetate, CH, . COOC6Hn . . 130*15 *8708/20° —— 140 „ alcohol, (CH3)2CH(CH2)2OH 88*12 *81/20° —134 131 Isobutane, (CH3)2CHCH, .... 58*10 — — 145° — 10*2 Isobutyl alcohol, (CH3)2CH . CH2QH 74*10 •800/18° — 108 *4 108*4 „ amine, (CH3)2CHCH2NH2 . 73*12 •736/15° — 85*5 68 Isobutyric acid, (CH3)8CH . COOH . 88*o8 •9516/20° —47 I55'5 Isopentane, (CH3)2CHCH2CH3 . . 72*12 / ’6393/0° 1 \ -6196/20° / - i58*5 27*9 Isopropyl acetate, CH8COOCH(CH3)8 „ alcohol, (CH3)2HC(OH) 102*11 •917/0° — 73*4 90-93 60*08 •789/20° -85*8 82*8 d., dextro-rotatory (see p. 89) ; dec. or decomp. = decomposes; subl. Journ. de Phys., Jan., 1909. = sublimes ; Y., Young,](https://iiif.wellcomecollection.org/image/b31356904_0153.jp2/full/800%2C/0/default.jpg)