Tables of physical and chemical constants and some mathematical functions / by G.W.C. Kaye and T.H. Laby.
- G. W. C. Kaye
- Date:
- [1941]
Licence: Public Domain Mark
Credit: Tables of physical and chemical constants and some mathematical functions / by G.W.C. Kaye and T.H. Laby. Source: Wellcome Collection.
155/200 (page 143)
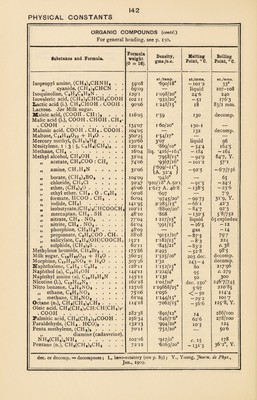
![PHYSICAL CONSTANTS ORGANIC COMPOUNDS [contd.) For general heading, see p. 130. Substance and Formula. Formula weight (0 = 16). Density, gms./c.c. Melting Point, 0 C. Boiling Point, 0 C. Phenetol, C6H6OCaH8. 122*12 at./temp. *963/25° 1*06/33° at./mms. -34 at./mms. 171 Phenol, C«H5 . OH. 94*08 427 l8l*5 Phenyl acetic acid, C6H6CH2COOH . I36*I 1*23 76-5 265 „ cyanide, CeH6CN. 103*09 I*008/l70 -17 190 „ hydrazine, C6H6HN.NH2 108*1 I 098/20° 19*35 243-5 Phloroglucin, 1:3: 5,C4H8(0H)32H20 162*11 — 218 anhy. sublimes Phthalic acid, 0. C„H4(COOH)2 . . „ anhydride, C6H4<(C0)2>0 166-09 i*59 180-200 — 148*07 i'53/4° 128 284 Picoline (0), CHS . C6H4N .... 93*10 *933/22° -69*9 129 Picric acid, 1:2:4: 6, C8H20H(N02)3 229 08 1767/19 122*5 explodes Pinene. See Turpentine. Propane, CHa . CH2 . CHa . . . . 44*08 *535 -187*8 “44*1 Propionic acid, CHa . CHa . COOH . Propyl acetate (n.), CH8COO . C8H7 . 74*06 *9870/20° “I9-3 140 102*11 •8884/20° -92-5 ioi*6 „ alcohol (n.),CHsCHaCHa. OH 60*08 *804/20° -127 97*2 „ chloride (n.), CH8CHaCPIaCl . 78-53 •891/18° - I22'8 46*5 „ formate, H . COO . C3H7 . . 88*08 *9058/20° -92*9 80 *9, Y. „ iodide, CHS . CH2 . CHaI . . I70*0 1745/20° - 101*4 102 Propylene, CHS . CH : CHa .... 42*06 A.4376 - 185*2 - 50*2 Pseudo-cumene, 1:2:4, CeH8(CH8)8 . 120*14 *8748/20° -57’4 169*8 Pyridine, C»H6N. 79*08 •985/15° -42 115*4 Pyrogallol (—ic acid, or “ pyro 1:2:3, C,Hj(OH),. 126*08 1*46/40° 133 293 Pyrrol, (CH)<>NH. 67*07 •967/21° liquid 131 Quinoline, C8H4<C^ * . . . 129*11 1 *094/20° — 22*6 241 Quinine, CaoHa4NaOa. 324-31 — anhy. 174*9 — „ sulphate, (C20H24N2O2)2 .- H2S04 + 7H20 . . 872*81 1- 205, dry — Racemic acid, (COOH . CH(OH))a- + HaO.. 168 08 1*69/7° 205 _ Rochelle salt (d.), KNaC4H40@.4HaO 282*22 117 — — Rosaniline (p.), (C6H4NH2')3COH . . 305*28 188-9 — Saccharin, CeH4< C0S02> NH . . 183*15 — 220 dec. — Salicylic acid, OH . C6H4 . COOH Sodium ethyl, NaC2H6. 138 08 1*48/4° 158/760 sublimes 52*05 2 7 — Stearic acid, CH3(CH2)16COOH . . . 284*38 ’843/80° 69*3 291/100 Stearine, (C18HS602)3CSHB. 891*16 •924/65° 71-1*5 — Succinic acid, COOH(CH2)2COOH . 118*07 1*564/15° 185 235 Sugar, cane-, ClaH22On. Sulphanilic acid (p.), NH2.C6HrS03H . 2H20. 342*24 1*5877/18° 189 — 209*18 chars 1 Sulphonal, (CH3)2C(S02C2H6)2 . . . 228*22 — 125 300 dec. Tartaric acid (i. or meso), COOH- [CHOH]2COOH . H20 168*08 1*67 142 anhy. - - — „ „ (d.), COOH(CHOH)2- COOH. 150*07 1*76/7° P. 170 „ „ „ (1.), COOH(CHOH)a- COOH. 150*07 1*76 170 — Terephthalic acid (p.), C6H4(COOH)a. 166*09 — sublimes —• Terpenol (y), C10H18O ...... 154*19 70 1 anhy. = anhydrous ; d. = dextro-rotatory (see p. 89) ; P., Perkin dec. — decomposes;](https://iiif.wellcomecollection.org/image/b31356904_0155.jp2/full/800%2C/0/default.jpg)