Clinical diagnosis a text-book of clinical microscopy and clinical chemistry for medical students, laboratory workers, and practitioners of medicine / by Charles Phillips Emerson.
- Emerson Charles Phillips, 1872-1938.
- Date:
- 1911
Licence: In copyright
Credit: Clinical diagnosis a text-book of clinical microscopy and clinical chemistry for medical students, laboratory workers, and practitioners of medicine / by Charles Phillips Emerson. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
75/808 (page 51)
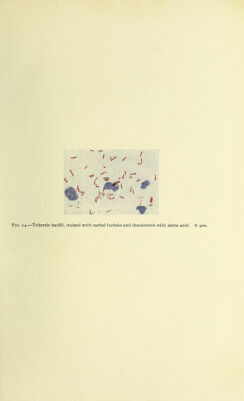
![specimen is first deeply stained with a penetrating dye which will stain all bacteria. It is then decolorized with an agent which will remove the stain from practically all organisms except Bacillus tuber- culosis. If the smear is then counterstained, the tubercle bacillus alone will retain the red color, while practically all the others will become blue. It is, however, difficult to get the tubercle bacillus to take any stain at all. The Ziehl-Neelsen carbolfuchsin mixture is the one in common use. (Fuchsin, i gm.; absolute alcohol, lo c.c.; 5 per cent, carbolic acid, 100 c.c.) The specimen can be stained'by heat or in the cold. If the latter is used, the specimen is left sub- merged in this stain for twenty-four hours. If heat is preferred the shde or cover-glass, whichever is used, may be submerged in a flat dish containing the stain and heated over a free flame; or the cover, held in a suitable forceps, and the slide, if that is used, held in a holder, or in the fingers, is covered with the stain and heated over the free flame. It is necessary to keep renewing the evaporating carbolfuchsin. The staining fluid must actually boil, or the tubercle bacilli will not take the stain. For it merely to steam is not sufficient. It is usually enough for it to boil from a quarter to half a minute; but, to be on the safe side, many prefer to let it boil from one to' four mmutes. With the best of technic probably but a fraction of the tubercle bacilli will take any stain; so that one must be careful that the mitial staining be as complete as possible. Since high heat certamly injures the specimen, if very careful work is desirable, the cold, slow method is to be preferred. There are several ways of decolorizing the specimen. The best reagent is acid alcohol [2 per cent. HCl (some say 3 per cent.) in 80 per cent, alcohol]. The slide may be dipped into a vessel of this fluid or the acid alcohol repeatedly poured on and drained off. The decolorizing process should be repeated until the specimen is to the eye practically colorless. It is hastened by warming the slide covered with the fluid. Many urge the watching of the process under the low power of the microscope, and this does enable one to determine very accurately when the specimen is sufficiently decolorized. Some advise that the specimen be decolorized with 25 per cent, nitric acid and in the very popular Gabbett's method 25 per cent, sulphuric acid is used. The latter burns the specimen more, and makes the bacilli look thicker, and their beading, etc., less distinct than does nitric acid. The disadvantage of these two acids is that there are many acid-fast bacilli, several of which might be encountered in the sputum. These any method involving the use of acid as decolorizing agent would not differentiate from the tubercle bacillus. On the other hand, there are few acid-fast and alcohol-fast bacilli For this](https://iiif.wellcomecollection.org/image/b21699550_0075.jp2/full/800%2C/0/default.jpg)