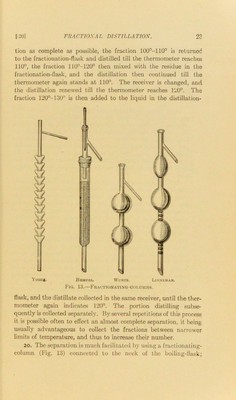
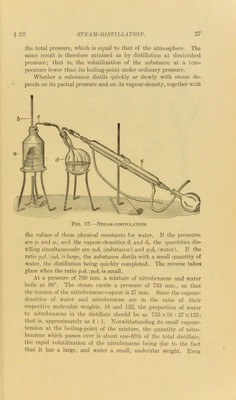
A text-book of organic chemistry / by A.F. Holleman ; edited by A. Jamieson Walker ; assisted by Owen E. Mott ; with the co-operation of the author.
- Arnold F. Holleman
- Date:
- 1913
Licence: In copyright
Credit: A text-book of organic chemistry / by A.F. Holleman ; edited by A. Jamieson Walker ; assisted by Owen E. Mott ; with the co-operation of the author. Source: Wellcome Collection.
45/646
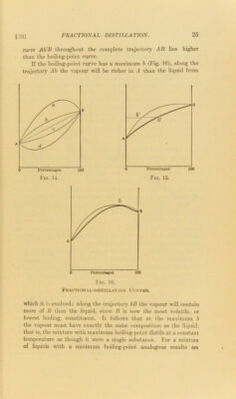
![§21] curve Ab'B throughout the complete trajectory AB lies higher than the boiling-point curve. If the boiling-point curve has a maximum b (Fig. 16), along the trajectory Ab the vapour will be richer in .4 than the liquid from Fractional-distillation Curves. which it h evolved: along the trajectory bB the vapour will contain more of II than the liquid, since B is now the most volatile, or lowest boiling, constituent. It follows that at ihe maximum b the vapour must have exactly the same composition as the liquid; that, is, the mixture with maximum boiling-point distils at a constant temperature as though it were a single substance. For a mixture of liquids with a minimum boiling-point analogous results are](https://iiif.wellcomecollection.org/image/b28134424_0045.jp2/full/800%2C/0/default.jpg)