A text-book of organic chemistry / by A.F. Holleman ; edited by A. Jamieson Walker ; assisted by Owen E. Mott ; with the co-operation of the author.
- Arnold F. Holleman
- Date:
- 1913
Licence: In copyright
Credit: A text-book of organic chemistry / by A.F. Holleman ; edited by A. Jamieson Walker ; assisted by Owen E. Mott ; with the co-operation of the author. Source: Wellcome Collection.
593/646
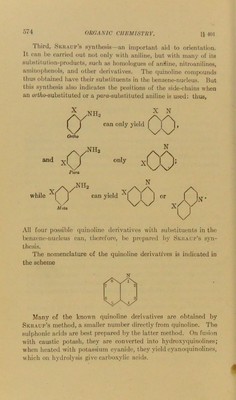
![§ 401] QUINOLINE. the reduction of o-nit rocinnamaldehvde. This compound is first converted into an intermediate product, the corresponding amino- derivative, the H-ntoms of the NH2-group of this substance being subsequently eliminated along with the O-atom of the aldehyde- group: o-AminocinnamaMehydo Quinoline The last synthesis proves quinoline to be an ort/to-substituted benzene: the constitution of the ring containing the N-atom has now to be determined. The method employed is based upon oxi- dation, which produces a dibasic acid, quinolinic acid, N HOOC/^.H HOOc’X/Jrr H On distillation with quicklime, quinolinic acid yields pyridine. From these facts it must be concluded that quinoline contains a benzene-nucleus and a pyridine-nucleus, with two ortho-C-atoms common to both. It may be regarded as naphthalene, with one of the CH-groups, 1-4-5-8, replaced by N. The number of isomeric substitution-products is very large. The seven hydrogen atoms occupy dissimilar positions relative to the nitrogen atom, and consequently seven monosubstitution- products are possible. Twenty-one disubstitution-products are possible for similar substituents, while the number of tri-derivatives possible is much greater, and so on. 401. There arc three methods for the orientation of quinoline derivatives. First, the relative method (354, 1). Second, oxidation. This process usually removes the benzene- nucleus, leaving the pyridine-nucleus intact, and thus furnishes a means of determining which substituents are present in each.](https://iiif.wellcomecollection.org/image/b28134424_0593.jp2/full/800%2C/0/default.jpg)