A text-book of organic chemistry / by A.F. Holleman ; edited by A. Jamieson Walker ; assisted by Owen E. Mott ; with the co-operation of the author.
- Arnold F. Holleman
- Date:
- 1913
Licence: In copyright
Credit: A text-book of organic chemistry / by A.F. Holleman ; edited by A. Jamieson Walker ; assisted by Owen E. Mott ; with the co-operation of the author. Source: Wellcome Collection.
595/646
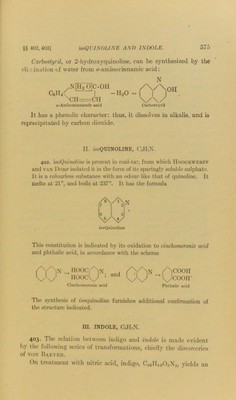
![§§ 402, 403] Carbostyril, or 2-hydroxyquinoline, can be synthesized by the eli m ination of water from o-aminocinnamic acid: C6H ^NfHTOjC-OH 1\ih= rCH o-Amicocinnamic acid -h2o N Carbostyril It has a phenolic character: thus, it dissolves in alkalis, and is reprecipitated by carbon dioxide. II. isoQUINOLINE, C,II7X. 402. isoQuinoline is present in coal-tar, from which IIoogewerff and van Dorp isolated it in the form of its sparingly soluble sulphate. It is a colourless substance with an odour like that of quinoline. It melts at 21°, and boils at 237°. It has the formula isoQuinoline This constitution is indicated by its oxidation to cinchomcronic acid and phthalic acid, in accordance with the scheme hooc/Nn. HOOC^y ’ and Cinehomeronic acid /Ncooh k^ycooir Phthalic acid The synthesis of woquinoline furnishes additional confirmation of the structure indicated. III. INDOLE, C,H7N. 403. I'he relation between indigo and indole is made evident by the following series of transformations, chiefly the discoveries of von Baeyeh. On treatment with nitric acid, indigo, C1GH10O2N2, yields an](https://iiif.wellcomecollection.org/image/b28134424_0595.jp2/full/800%2C/0/default.jpg)