A text-book of organic chemistry / by A.F. Holleman ; edited by A. Jamieson Walker ; assisted by Owen E. Mott ; with the co-operation of the author.
- Arnold F. Holleman
- Date:
- 1913
Licence: In copyright
Credit: A text-book of organic chemistry / by A.F. Holleman ; edited by A. Jamieson Walker ; assisted by Owen E. Mott ; with the co-operation of the author. Source: Wellcome Collection.
597/646
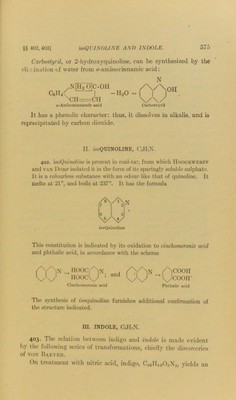
![§ 103] NH Indole Indole, therefore, possesses a benzene-nucleus condensed with a pyrrole-nucleus. It does, in fact, display some of the properties characteristic of pyrrole: thus, it is a very weak base, and gives a red coloration with hydrochloric acid. W-M ethyl indole, or scatole, NH C8Il/ )CH, c«ch3 is present in f;eoes, and occasions the unpleasant odour. It is also found in a species of wood grown in India, and is formed in the putrefactive decay of proteins, or by fusing proteins with caustic potash. Tryptophan or indolealaninc, CnHi202N2, is an important decom- position-product of proteins (252, 5) and an indole derivative. It is synthesized by treating indole with chloroform and potassium hydroxide in alcoholic solution. 3-Indolealdehyde (I.X is formed as an intermediate product, and condenses with hippuric acid to indolyl- benzoylaminoacrylic acid (II.). On treatment with sodium and alcohol, the double bond of this compound adds two hydrogen atoms and the benzoyl-group is simultaneously eliminated, with formation of racemic tryptophan (III.): NH Tryptophan III.](https://iiif.wellcomecollection.org/image/b28134424_0597.jp2/full/800%2C/0/default.jpg)