Introduction to the study of organic chemistry : the chemistry of carbon and its compounds / by Henry E. Armstrong.
- Henry Edward Armstrong
- Date:
- 1890
Licence: Public Domain Mark
Credit: Introduction to the study of organic chemistry : the chemistry of carbon and its compounds / by Henry E. Armstrong. Source: Wellcome Collection.
349/378 (page 327)
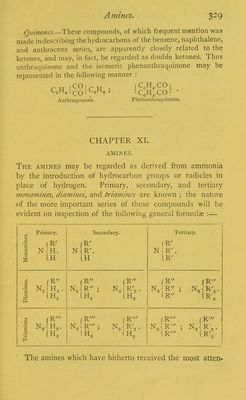
![2. The ketones are especially characterised by their be- haviour on oxidation— Law of Oxidation of the Ketones.—The law may be stated in the following general terms :—On oxidising the ketones one of the hydrocarbon groups in combination with the CO group is converted by the assumption of OH into the corresponding monobasic acid ; the other is split off and separately oxidised. It appears that it is always the less stable (usually the more complex) hydrocarbon group which is thus split off. In the case of the ketones of the CO(CnH2n+1)2 series, which furnish acids of the acetic series on oxidation, if this group is derived from a primary alcohol it is converted into the acid of the acetic series containing the same number of units of carbon :l Methyl-propyl ketone, for example, yields acetic and pro- pionic acids: If the hydrocarbon group is derived from a secondary alcohol it is oxidised to the corresponding ketone : Thus methyl-isopropyl ketone yields acetic acid and acetone: 1 Probably the oxidation does not take place at a single stage, but the ketone is perhaps in the first place resolved by the combined action of the nascent oxygen and water (or it may be by the action of hydric peioxide) into an acid and an alcohol in the manner indicated by the equations: C°(ch!(C!Hs) + 3° = CHlCQ(OH) + C„HsCO(OH). C H C01 CH(CH3)2 + 20 = CH3CO(OH) + CO(CH3)2. j + (O + OH2) = CnPI2n+1CO(OH) + CmH2m+]. CH2(OH); )o + (O + OH2) = CnH2n+1CO(OH) *■ (f-mHam+ila- CH(OH); the alcohol thus formed being at once further oxidised.](https://iiif.wellcomecollection.org/image/b21499445_0349.jp2/full/800%2C/0/default.jpg)