The elements of thermal chemistry / by M.M. Pattison Muir ; assisted by David Muir Wilson.
- M. M. Pattison Muir
- Date:
- 1885
Licence: Public Domain Mark
Credit: The elements of thermal chemistry / by M.M. Pattison Muir ; assisted by David Muir Wilson. Source: Wellcome Collection.
324/338
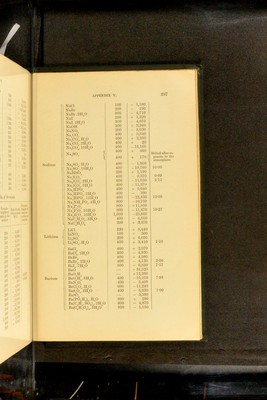
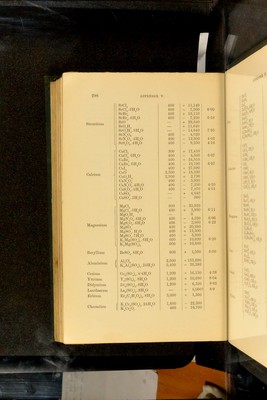
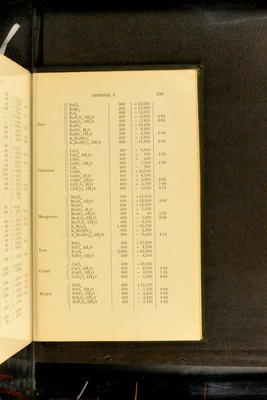
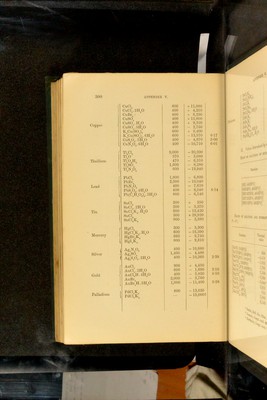
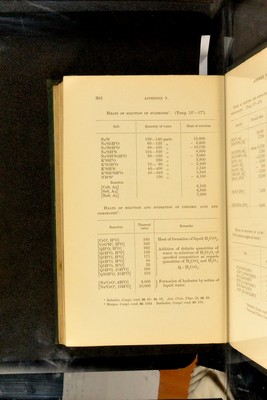
![Salt Quantity of water Heat of solution Na®S 130—140 parts 15,000 NaS5H-°0 60—120 „ - 6,600 NaS9H”0 60—105 „ -16,720 Na”SH-S 104-830 „ 8,800 NaSH“S4H0 60—100 „ • - 3,060 KSH-0 230 „ 3,800 K“S5H=0 75— 90 „ - 5,200 K”SHS 40—400 „ 1,.540 KSHSH-0 45—240 „ 1,340 NH^S 150 „ - 4,100 Eeaction 'CaS, Aq] 6,100 'SrS, Aq] 'BaS, Aq] 6,800 7,000 Heats of solution and iiydkation of cnROMic acid and NIROMATES^ Eeaction Thermal value Eemarks rciO”. ff 0] 580 Heat of formation of liquid H^CrO^. ■CrO^H% H^’O] 340 ■QffO, ffO] 260 Addition of definite quantities of ■Q2H”0, ff 0^ 135 water to solutions of H^Or^O^ of ■Q3H-0, H0 171 „ .'^pecihed composition as regards ■Q4ff 0, H-0 80 quantities of H^UrO^ and H^O ; ■Q5ff 0, ffO 35 0 - H CrO . ■Q5H-0, 25H‘ 0] 500 ■Q30H”O, 25H*0] 210 [NaCrO\ 4H“0] 9,800 1 Formation of hydrates by action of ;Na”CrO^ 10H”O] 18,000 J liquid water. 1 Sabatier, Compt. rend. 89. 43: 91. 52. Ann. Chim. Phys. (5). 22. 98. - Merges, Coin^t^ rend, 86. 1444 Bertlielot, Compt. lend. 87. ol 802 APPENDIX V. Heats of solution op sulphides'. (Temp. 15”—17”).](https://iiif.wellcomecollection.org/image/b28065050_0324.jp2/full/800%2C/0/default.jpg)