The elements of thermal chemistry / by M.M. Pattison Muir ; assisted by David Muir Wilson.
- M. M. Pattison Muir
- Date:
- 1885
Licence: Public Domain Mark
Credit: The elements of thermal chemistry / by M.M. Pattison Muir ; assisted by David Muir Wilson. Source: Wellcome Collection.
326/338
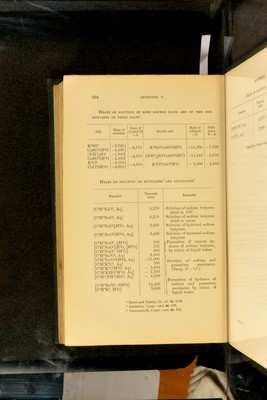
![Heats op solution of butyrates® and succinates®. Eeaction Thermal value Eemarks ^H'NaO®, Aq] i^H'NaO®, Aq] !‘H'NaO®^H®0, Aq] !^H'Na0®3H®0, Aq] )‘H'NaO®, 1H®0] ;^H'NaO®|H®0, l-ffO] rH’NaO®, 3H®0] rH‘J^a®0\ Aq] yH'Na®0^6H®0, Aq] Aq] Aq] :^H^KH0^H®0, Aq] :!•'HXNH•‘)HO^ Aq] :;^HNa®0^ 6H®0] H®0] 4,270 4,210 3,660 3,440 580 220 800 8.400 11,000 200 - 3,400 - 7,600 - 4,900 19.400 3,600 Solution of sodium Imtyrate dried at 100. Solution of sodium butyrate dried in vacuo. Solution of hydrated sodium butyrate. Solution of hydrated sodium butyrate. I Formation of various hy- I- drates of sodium butyrate, ) by action of liquid water. Solution of sodium and potassium succinates. (Temp. 8 - 11). (■Formation of hydrates of sodium and potassium succinates by action of liquid water. 1 Favre and 'Valson, loc. cit. 73. 1150. • Berthelot, Compt. rend. 80. 512. 3 niiroustchoff. Compt. rend. 89. 570. 04 APPENDIX V. Heats op solution of some double salts, and of the con- riTUENTS OF THESE SALTS'. Salt Heat of solution Sum of (l)and(2) = A Double salt Heat of solution = B. Diffe- rence B-A -6,3401 -2,430 ( -1,940\ -2,430) ,1501 ,650/ -9 -f4, -8,770! K®S0^CuS0^6H®0 -4,370 -4,500 ; (NH^),S0^CuS0^6H®0 K®Cl®CuCPH®0 -14,360 -11,240 - 6,980 -5,590 - 6,870 - 2,480 10^5H®0 r),so^ 10^5H®0 Ip 5f2H®0](https://iiif.wellcomecollection.org/image/b28065050_0326.jp2/full/800%2C/0/default.jpg)