The fuel of life : experimental studies in normal and diabetic animals / by John James Rickard Macleod.
- Macleod, J. J. R. (John James Rickard), 1876-1935.
- Date:
- 1928
Licence: In copyright
Credit: The fuel of life : experimental studies in normal and diabetic animals / by John James Rickard Macleod. Source: Wellcome Collection.
145/168 (page 129)
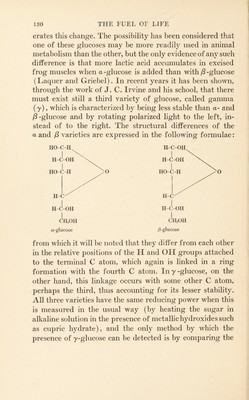
![from the sugar supplied by the blood, glycogen never again returns to sugar within the muscle itself, but is converted into lactic acid. To this extent it has entered an irreversible reaction, thus: Glucose -> glycogen ^ lactic acid. On the other hand when the glycogen of the liver breaks down glucose rather than lactic acid is formed, and the question arises as to whether this glucose is exactly of the same nature as that out of which the glycogen was formed. That the muscles can use glucose which has not gone through a glycogen stage in the liver is shown by numerous facts, among which the following may be mentioned: (1) the immediate effect of injections of glucose in removing the symptoms of hypoglycaemia even in the absence of the liver; (2) the increased respiratory exchange (O2 con- sumption) in the isolated heart, in eviscerated cats and in hepatectomized dogs when glucose is injected. It may be in all these cases that the foreign glucose passes through a glycogen stage in the muscles themselves before it is actu- ally used, a view which receives some support in the fact that glycogen is formed in the muscles when glucose is injected into eviscerated cats along with insulin (Best, Hoet, and Marks) and also, to a less extent, in intact ani- mals without the injection of insulin (Choi). In any case, the possibility has to be borne in mind that some definite change may occur in the glucose molecule itself preceding its entry into any one of these processes, and it is with a consideration of this possibility that I bring the present chapter to a close. As is well known, glucose when dissolved in water exists at first as a -glucose with a high rotatory power ap] 110° but soon changes in part into another variety, ^-glucose, with a low rotatory power, apj 19°. This causes the rotatory power of the solution gradually to fall until a stable mixture of the two varieties, giving a a of 52.5°, is formed. The addition of weak alkali greatly accel-](https://iiif.wellcomecollection.org/image/b29807608_0145.jp2/full/800%2C/0/default.jpg)