The preparation and some properties of purified diphtheria toxoid / by Arthur Frederick Watson and Elsie Langstaff.
- Watson, A. F.
- Date:
- [1926?]
Licence: In copyright
Credit: The preparation and some properties of purified diphtheria toxoid / by Arthur Frederick Watson and Elsie Langstaff. Source: Wellcome Collection.
6/20 (page 766)
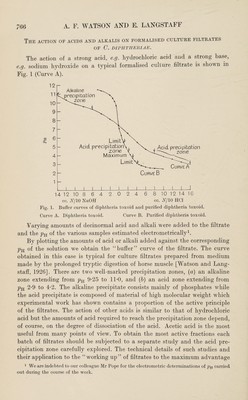
![The action of acids and alkalis on formalised culture filtrates OF C. DIPHTHERIAE. The action of a strong acid, e.g. hydrochloric acid and a strong base, e.g. sodium hydroxide on a typical formalised culture filtrate is shown in Fig. 1 (Curve A). cc. N/10 NaOH cc. N/10 HC1 Fig. 1. Buffer curves of diphtheria toxoid and purified diphtheria toxoid. Curve A. Diphtheria toxoid. Curve B. Purified diphtheria toxoid. Varying amounts of decinormal acid and alkali were added to the filtrate and the pH of the various samples estimated electrometrically1. By plotting the amounts of acid or alkali added against the corresponding pH of the solution we obtain the “buffer” curve of the filtrate. The curve obtained in this case is typical for culture filtrates prepared from medium made by the prolonged tryptic digestion of horse muscle [Watson and Lang- staff, 1926]. There are two well-marked precipitation zones, (a) an alkaline zone extending from pH 9-25 to 11*0, and (b) an acid zone extending from pH 2*9 to 4-2. The alkaline precipitate consists mainly of phosphates while the acid precipitate is composed of material of high molecular weight which experimental work has shown contains a proportion of the active principle of the filtrates. The action of other acids is similar to that of hydrochloric acid but the amounts of acid required to reach the precipitation zone depend, of course, on the degree of dissociation of the acid. Acetic acid is the most useful from many points of view. To obtain the most active fractions each batch of filtrates should be subjected to a separate study and the acid pre¬ cipitation zone carefully explored. The technical details of such studies and their application to the “working up” of filtrates to the maximum advantage 1 We are indebted to our colleague Mr Pope for the electrometric determinations of pH carried out during the course of the work.](https://iiif.wellcomecollection.org/image/b30625385_0006.jp2/full/800%2C/0/default.jpg)