On the physiological action of carbon monoxide of nickel / John G. McKendrick and William Snodgrass.
- McKendrick, John G. (John Gray), 1841-1926.
- Date:
- [1891]
Licence: Public Domain Mark
Credit: On the physiological action of carbon monoxide of nickel / John G. McKendrick and William Snodgrass. Source: Wellcome Collection.
Provider: This material has been provided by The University of Glasgow Library. The original may be consulted at The University of Glasgow Library.
13/18 (page 11)
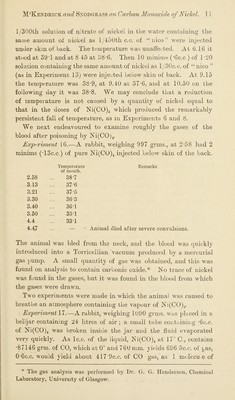
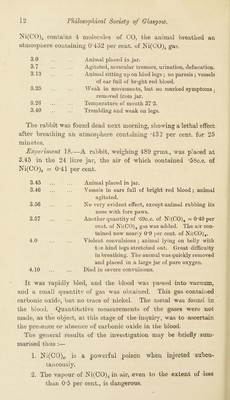
![l/300th solution of nitrate of nickel in the water containing the same amount of nickel as l/45Utlic.c. of “ nicowere injected under skin of back. The temperature was unalfected. At 6.16 it stood at 39T and at 8.45 at 38*6. Then 10 minims (*6c.c ) of 1-20 solution containing the same amount of nickel as l/30c.c. of “ nico ” (as in Exjieriment 13) were injected below skin of back. At 9.15 the temperature was 38*9, at 9.40 at 37*6, and at 10.50 on the following day it was 38*8. We may conclude that a reduction of temperature is not caused by a quantity of nickel equal to that in the doses of Ni(CO)4 which produced the remarkably persistent fall of temperature, as in Experiments 6 and 8. We next endeavoured to examine roughly the gases of the blood after poisoning by ]Sii(CO)4. Exp*^riment 16.—A rabbit, weighing 997 grins., at 2*58 had 2 minims (*13c.c.) of pure Ni(CO)4 injected below skin of the back. 2.58 Temperature of mouth. 38*7 3.13 37*6 3.21 37*5 3.30 36*3 3.40 36*1 3.50 35*1 4.4 33*1 4.47 ... Remarks. Animal died after severe convulsions. The animal was bled from the neck, and the blood was quickly introduced into a Torricellian vacuum produced by a mercurial gas pump. A small quantity of gas was obtained, and this was found on analysis to contain carbonic oxide.* INo trace of nickel was found in the gases, but it was found in the blood from which the gases were drawn. Two experiments were made in which the animal was caused to breathe an atmosphere containing the vapour of iN 1(00)4. Experiment 17.—A rabbit, weighing 1U90 grms. was placed in a belljar containing 24 litres of air; a small tube containing *6c.c. of Ni(CO)4 was broken inside the jar and the fluid evaporated very quickly. As Ic.c. of the liquid, Ki(CO)4 at 17° C., contains *87146 grm. of CO, which at 0° and 760 mm. yields 696 ^c.c. of gas, 0*6c.c. would vield about 417 9c.c. of CO <^as, as 1 molecuie of y O' * The gas analysis was performed by Dr. G. G. Henderson, Chemical Laboratory, University of Glasgow.](https://iiif.wellcomecollection.org/image/b24930155_0015.jp2/full/800%2C/0/default.jpg)