A study of the phenomena and causation of heat-contraction of skeletal muscle / by T.G. Brodie and S.W.F. Richardson ; communicated by W.D. Halliburton.
- Brodie, Thomas Gregor, 1866-1916.
- Date:
- 1899
Licence: Public Domain Mark
Credit: A study of the phenomena and causation of heat-contraction of skeletal muscle / by T.G. Brodie and S.W.F. Richardson ; communicated by W.D. Halliburton. Source: Wellcome Collection.
Provider: This material has been provided by The University of Glasgow Library. The original may be consulted at The University of Glasgow Library.
13/24 page 135
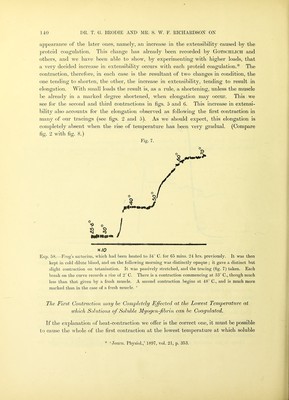
![The maximum effect is produced at 35° C. The coagulation-temperature is markedly affected by the quantity of salt present in the solution. (6.) A nucleo-proteid (Pekelharing*) which is only present in small quantities. In the muscle-plasma of the rabbit there is one very important difference, viz., that soluble myogen-fibrin, if present at all, is only present in quite minimal quantities. The main proteids, if not the only ones characteristic of muscle, are then myogen and myosin (v. Furth), and in addition for frog’s muscle-plasma, soluble myogen-fibrin. Another important point relates to the formation, from these proteids, of coagulated varieties, i.e., forms of proteid insoluble in the ordinary saline media. According to V. Furth, the change which myosin (paramyosinogen) undergoes is directly into an insoluble form, which he terms myosin-fibrin. For myogen (myosinogen) the change is more complex. Ajiparently myogen, in the first instance, becomes changed into a soluble proteid, viz., the soluble myogen-fibrin we have already described. This change is remarkably rapid at about 40° C., but much less so at a slightly lower temperature. Soluble-myogen-fibrin, in its turn, is converted into an insoluble proteid, myogen-fibrin, and thus the series of changes of myogen are comjileted. In addition to these proteids there are still other chemical substances in muscle to be taken into account when we are studying the effect of heat upon muscle. These are the fibrous tissue forming the peri- and endo-mysium and the sarcolemma sheaths. The sarcolemma is a substance which has been shown by EwALof to closely resemble elastin in its solubilities. Halliburton describes a proteid of the globulin class, giving a heat-coagulum at 75° C., as being present in the ground-substance of connective tissue. With regard to the influence of heat upon tendon and upon elastic tissue, we have in the first place the work of Hermann,;]; who found that tendon began to contract at 65° C. and finished at 75° C. In this connection we reproduce a tracing we have obtained from a thin tendon taken from the mouse’s tail (fig. 4), from which it is seen that the contraction begins at 60° C., is complete at about 64° C., and is followed by a rapid elongation terminating in rupture soon after 65° C. The load in this experiment was about 1 gram. The influence of heat upon elastic tissue has been studied, among others, by Gotschlich,§ who found that a piece of ligamentum nuchae shortened on heating and lengthened on cooling for all temperatures up to 65° C. Between 65° C. and 75° C. another contraction occurs which does not disappear on cooling. A closely analogous behaviour was observed by him[| in a muscle which had been previously brought by heat into a condition of heat-rigor. * ‘ Zeitschr. f. Physiol. Chem.,’ vol. 22, p. 245, 1896. t ‘ Zeitschr. f. Biol.,’ vol. 26, p. 1, 1889. J Loc. cit. § Loc. cit., p. 116. II Pfluger’s ‘ Archiv,’ vol. 55, p. 339, 1894.](https://iiif.wellcomecollection.org/image/b24917230_0015.jp2/full/800%2C/0/default.jpg)