Licence: Public Domain Mark
Credit: Quantitative chemical analysis / by C. Remigius Fresenius. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
158/436 (page 146)
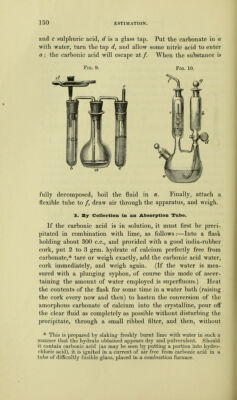
![]46 Treat the dry mass at the common temperature with acetate of potassium solution (1 in 4), allow to stand a few hours, with frequent stirring, then decant the fluid onto a weighed filter, and wash the precipitate, first by decantation and then on the filter, with acetate of potassium, until the washings are no longer precipitated by chloride of calcium. In this manner, all the fluoride of hydrogen and potassium is re- moved, without any of the borofluoride of potassium being dissolved. Finally, wash the precipitate with alcohol of •85 s.g., dry at 100°, and weigh. Chloride, nitrate, and phosphate of potassium, and salts of sodium, dissolve in acetate of potassium. Sulphate of potas- sium dissolves with some difficulty. Salts of sodium, how- ever, must not be present in large quantity, as fluoride of sodium is only soluble with great difficulty. Stromeyer's test-analyses gave from 97'5 to 1007 instead of 100. As the borofluoride of potassium is very likely to contain silicofluoride of potassium, it is indispensable to test it for this substance. This is done by placing a portion on moist litmus paper, and another portion in cold strong sulphuric acid. If the paper is reddened, and the acid produces effervescence, silicofluoride of potassium is present. To remove it, dissolve the remainder of the salt, after weighing it, in boiling water, add ammonia, and evaporate; redissolve in boiling water, add ammonia, &c., repeating the operation several times. Finally, after warming once more with ammonia, filter off the silicic acid, evaporate to dryness, and treat again with acetate of potassium and alcohol. Carbonic Acid. 1. By standard Lime Water. (For very weak solutions.) 2. By loss of Weight. On ignition. • On fusion with borax. On treatment with acids. 3. By collection in weighed Absorption-tube. 4. By measurement of the Gas. 1. By Standard Iiime Water. The carbonic acid water is mixed with a measured quantity of standard lime water in excess. After the carbonate of cal-](https://iiif.wellcomecollection.org/image/b21966989_0158.jp2/full/800%2C/0/default.jpg)