Volume 1
A dictionary of applied chemistry / by T.E. Thorpe ; assisted by eminent contributors.
- Thorpe T. E. (Thomas Edward), Sir, 1845-1925.
- Date:
- 1890-1893
Licence: Public Domain Mark
Credit: A dictionary of applied chemistry / by T.E. Thorpe ; assisted by eminent contributors. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
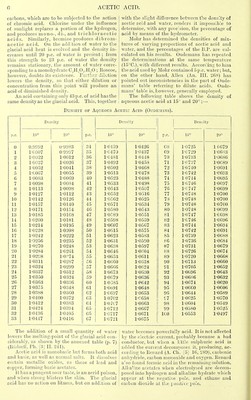
21/734
![So' idifj iug point -C Water to lOil parts real acetic aciil Solidifying point °G Water to lUU parts real acetic acid + i(;-70 00 + C-25 8-0 i(;(i5 0-5 5-30 9-0 14-80 10 4-30 100 li-OO 1-5 3-00 110 1.!'25 2-0 2-70 12-0 ll'J5 30 -0-20 150 10-.)0 4-0 -2-(10 18-0 9-40 5-0 -5-10 21-0 8-20 G-0 -7-40 24-0 7-10 7-0 Acetic acid mixes ■with alcohol and ether in all proportions. It dissolves resins, gelatin, fibrin, albumen, essential oils, etc. Phosphoi'us and sulphur are somewhat soluble in the warm acid. Valenta (D. P. J. 252, 29(3-297) and others have used it on account of this solvent power in the analysis of oils. Acetic acid is largely used in the preparation of tlie acetates of copper, aluminium, iron, lead, Ac, as pyroligncous acid in calico ])rinting; in the prepa'/ation of varnishes and colouring matters ; in the laboratory and certain industries for the solution of hydrocarljons and like sub- stances ; for domestic use ; in photography ; and in medicine as a local irritant and to allay fever, and in the form of smelling salts. Analysis.—Commercial glacial acid should contain at least 97 p.c. of absolute acid. If 9 volumes oil of turpentine be agitated with 1 volume of acid, no turbidity will be produced if the acid contain 97 p.c. or upwards. Acid of l>9-5 p.c. produces no turbidity with any propor- tion of tuipentine (Bardy, C. N. 40, 78). A very delicate test for the presence of water is to mix the acid with an equal bulk of bisul- phide of carbon in a dry tube, and warm with the hand for a few minutes ; in presence of a trace of water the liquid becomes turbid. The commercial acid is liable to contain sul- phuric acid, sulphates, sulphurous acid, hydro- chloric acid, chlorides, arsenic (derived from sulphuric acid), and copper, lead, zinc, and tin derived from the vessels used in the manu- facture. The presence of sulphuric acid or sulphates is shown by the production of a white precipi- tate with Ijarium chloride. To the filtered so- lution bromine or chlorine water is added, pro- ducing, if sulphurous acid be present, a further precipitate of barium sulphate. Hydrochloric acid and chlorides are detected and estimated with silver nitrate. In testing for metals a considerable bulk should bo evaporated ; if much organic matter be liresont, the liquid should be evaporated to dry- ness and ignited, and the residue dissolved in hydrochloric acid, a few drops of hydrochloric acid are added, and a current of sulphuretted hydrogen gas passed through the liquid ; a black or brown colouration or precipitate indicates lead or copper. Copper may also be detected in the evaporated liquid by the brown precipitate ])rodnccd on the addition of fcrrccyanitle of piitash, and estimated by electro-deposition. To test for zinc, the solution, after the pas- sage of sulphiiretted hydrogen, is liltered, nearly neutralised with ammonia, and acetate of soda added, when zinc will be precipitated as white sulphide. For arsenic Reinscli's test may be used. Small quantities of acetic acid may be recog- nised by neutralising with caustic potash, adding arsenious oxide, evaporating to dryness, and heating, when the characteristic odour of eaco- dyl is evolved. To determine the free acetic acid in a solu- tion it is usual to titrate a weighed quantity with a solution of caustic soda standardised witli a solution of acetic acid of known strength, or of hydric potassic tartrate (Stillwell and Gladding). As indicator litmus may be used, but as it is rendei'ed blue by the normal acetate of soda, it is preferable to use phenol-plithalein, to which that substance is neutral; tliis is also more delicate, and, where the licjuid is coloured, may be considerably diluted without impan-ing the delicacy of the reaction. To estimate small percentages of water in acetic acid, the melting-point may be determined and the percentage found by the table before given. Acetates, when heated alone, usually evolve acetone ; heated with sulphuric or phosplioric acid they evolve acetic acid; heated witli arse- nious acid cacodyl is produced. The acetic acid in acetates may be deter- mined by distilling about 1 gram (15 grains) of the salt nearly to dryness with 10 c.c. of a 40 p.c. solution of phosphoric acid (free from nit' ic and otlier volatile acids); water is added and the distillation repeated to remove tlie last traces of acetic acid; the distillates are mixed and titrated as above with standard alkali. This method of distillation may also be used for highly coloured solutions of acetic acid where direct titration is inadmissible. Preparation of Vinegar. In all processes forthe manufacture of vinegar advantage is taken of the oxidising action of tlie vinegar fungus already described ; the souring of wines and other alcoliolic liquids is due to tliis organism, the germs of which are always present in the air and are deposited, and grow in any suitable medium. The action is more rapid when the li(]uid is rich in vegetable matter and poor in alcohol, and when the surface exposed to the air is large. The percentage of alcohol should not, however, be too low ; the acetous fermentation proceeds but slowly in a liquid containing less than 3 p.c. alcohol. V/ine vinegar. Fr. Vinaign'; Gcr. Wcinrr.sig. In the great wine dittrict of Orleans, wines which have become sour are generally used for the prejiaration of vinegar. For this purpose full- bodied wines are pieferred. If they contain above 10 p.c. alcoh 1 they are usually diluted or mixed with weaker wines, so as to contain about that jjerccntage. The wine, before being fer- mented, is usually left for some time in contact with beech shavings, on which the lees are de- posited, rendering the wine bii;;liter. A certain amount of extractive matter is, however, in ecs- sary for the proper growth of the plant, and if the wine bo old and the matter deposited, the](https://iiif.wellcomecollection.org/image/b21713595_0001_0021.jp2/full/800%2C/0/default.jpg)