Foods, their composition and analysis : a manual for the use of analytical chemists and others : with an introductory essay on the history of adulteration / by Alexander Wynter Blyth.
- Date:
- 1882
Licence: Public Domain Mark
Credit: Foods, their composition and analysis : a manual for the use of analytical chemists and others : with an introductory essay on the history of adulteration / by Alexander Wynter Blyth. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
117/674 (page 83)
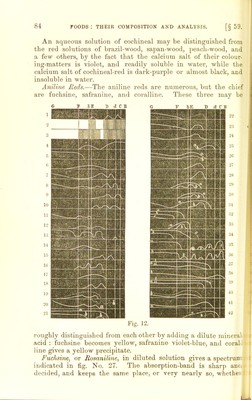
![§59.] 4. Insoluble in water and aloohol. He next subdivides his main divisions accoi'ding to the action of bisulphite of soda. The organic colouring-matters most likely to be found may be treated of in the order of the spectrum, beginning with the red. § 59. Reds.—The common reds are—cochineal, aniline reds, alkanet, and the madder-colours alizarine and purpurine. Cochineal.—Cochineal is a red complex colouring - matter, secreted by certain species of a peculiar family of insects feeding on the Cactus coccinellifera, C. spuntia, C. tunia, C. pereskia. The chief colouring-matter of cochineal is “ carminic acid,” the formula of which appears to be C17HlgO10. By the action of dilute acids carminic acid splits up into sugar, and a beautiful colour known as carmine-red, thus— Carminic acid. Water. Carmine-red. Glncose. Ci7H18O10 + 2HaO — CiiHjoOy + CbH10O5. Cochineal imparts its colouring-matters both to alcohol and water, and is precipitated by acetate of lead, carminate of lead being one of the constituent parts of the precipitate. The solu- tions of cochineal are purplish-red to crimson, turning a more or less rich violet-purple, with alkalies becoming of a yellow colour on the addition of acids. The colour is well known to chemists, as it is much used as an indicator for acids, being especially use- ful in titrating an alkaline liquid containing carbonates, since carminic acid is not affected by carbon dioxide like so many other colouring-m atters. Cochineal in neutral solutions gives absorption-bands, but not very definite when examined by the spectroscope ; if, however, it be made ammoniacal, then there are bands which differ in position only slightly from the absorption-bands of blood. No. 18 (fig. 12) is a graphical illustration of the spectrum of cochineal in water; No. 19, in alcohol; and No. 20, on the addition of nitric acid (a.) or N1I3 (6.). If alum is added to cochineal it loses its power of turning yellow with acids, and the purpurine band becomes so broad that the two bands almost run into each other. On addition of acetic acid they are separated, and appear as tolerably sharply-defined bands between D and E, and there is another at D. On dissolving cochineal with alum solution, a lake is obtained ; on dissolving this in tartaric acid, or dilute nitric acid, the solu- tion gives a band at B and E, and another close on D. The nitric acid solution gives a spectrum very similar to blood.](https://iiif.wellcomecollection.org/image/b2190165x_0119.jp2/full/800%2C/0/default.jpg)