The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins.
- Albert A. Hopkins
- Date:
- 1910
Licence: Public Domain Mark
Credit: The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins. Source: Wellcome Collection.
101/1096 page 87
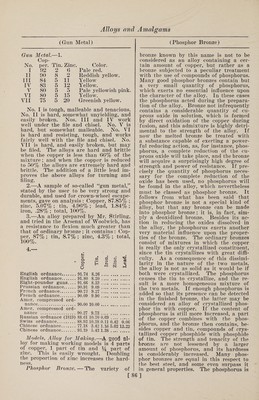
![fr Alloys and Amalgams (Phosphor Bronze) added to the bronze in the form of cop¬ per phosphide or phosphide of tin, the two being sometimes used together. They must be specially prepared for this pur¬ pose, and the best methods will be here given. Copper phosphide is prepared by heat¬ ing a mixture of 4 parts of superphos¬ phate of lime, 2 parts of granulated cop¬ per and 1 part of finely pulverized coal in a crucible, at a temperature not too high. The melted copper phosphide, containing 14% of phosphorus, separates on the bot¬ tom of the crucible. Tin phosphide is prepared as follows: Place a bar of zinc in an aqueous solu¬ tion of tin chloride. The tin will be sep¬ arated in the form of a spongelike mass. Collect it, and put it into a crucible upon the bottom of which sticks of phosphorus have been placed. Press the tin tightly into the crucible, and expose to a gentle heat. Continue the heating until flames of burning phosphorus are no longer ob¬ served on the crucible. The pure tin phosphide, in the form of a coarsely crys¬ talline mass, tin-white in color, will be found on the bottom of the crucible. To prepare the phosphor bronze the al¬ loy to be treated is melted in the usual way, and small pieces of the copper phos¬ phide and tin phosphide are added. Phos¬ phor bronze, properly prepared, has nearly the same melting point as that of ordi¬ nary bronze.. In cooling, however, it has the peculiarity of passing directly from the liquid into the solid state, without first becoming thickly fluid. In a melted state it retains a perfectly bright surface, while ordinary bronze in this condition is always covered with a thin film of oxide. If phosphor bronze is kept for a long time at the melting point there is not any loss of tin, but the amount of phos¬ phorus is slightly diminished. The most valuable properties of phosphor bronze are its extraordinary tenacity and strength. It can be rolled, hammered, and stretched cold, and its strength is nearly double that of the best ordinary bronze. It is principally used in cases where great strength and power of resistance to out¬ ward influences are required, as, for in¬ stance, in objects which are to be exposed to the action of sea water. Phosphor bronze containing about 4% of tin is ex¬ cellently well adapted for sheet bronze. With not more than 5% of tin it can be used, forged, for firearms ; 7 to 10% of tin gives the greatest hardness, and such bronze is especially suited to the manufac¬ ture of axle bearings, cylinders for steam fire engines, cogwheels, and, in general, (Phosphor Bronze) for parts of machines where great strength and hardness are required. Phosphor bronze, if exposed to the air, soon be¬ comes covered with a beautiful, closely adhering patina, and is, therefore, well adapted to purposes of art. The amount of phosphorus added varies from 0.25 to 2.5%, according to the purpose of the bronze. The composition of a number of kinds of phosphor bronze is given below : (1) Copper, 90.34% ; tin, 8.90% ; phos¬ phorus, 0.76%. (2) Copper, 90.86% ; tin, 8.56% ; phosphorus, 0.196%. (3) Cop¬ per, 94.71%; tin, 4.39%; phosphorus, 0.053%. (I) Copper, 85.55% ; tin, 9.85% ; zinc, 3.77%; lead, 0.62%; iron, traces; phos¬ phorus, 0.05%. (II) Tin, 4 to 15% ; lead, 4 to 15% ; phosphorus, 0.5 to 3%. (III) Tin, 4 to 15%; zinc, 8 to 20%; lead, 4 to 15% ; phosphorus, 0.25 to 2%. (IV) Copper, 77.85%; tin, 11%; zinc, 7.65%. (V) Copper, 72.50%; tin, 8%; zinc, 17%. (VI) Copper, 73.50%; tin, 6% ; zinc, 19%. (VII) Copper, 74.50% ; tin,' 11%; zinc, 11%. (VIII) Copper, 83.50% ; tin, 8% ; zinc, 3%. (I) for axle bearings, (II) and (III) for harder and softer axle bearings, (IV) to (VIII) for railroad purposes, (IV) especially for valves of locomotives, (V) and (VI) for axle bearings for wagons, (VII) for connecting rods, (VIII) for piston rods in hydraulic presses. Among other properties, phosphor bronze emits sparks under friction much less readily' than gun metal or copper, and oxidizes in sea water at about one- third the rate of copper. 1. —One of the principal uses of phos¬ phor bronze is in the form of springs. A good mixture for phosphor bronze springs is as follows: Copper, by weight, 95 parts ; tin, 4)4 parts ; 5% phosphor tin, y2 part. 2. —For phosphor bronze of the highest possible strength the following mixture is recommended : Copper, 90 parts ; tin, 9 parts; 5% phosphor tin, 1 part. The mixture made according to this formula is poured into ingots, and then remelted and poured into sand castings. The remelting increases the strength. 3. —For ordinary work, when a me¬ dium strength is required, and when scrap must be used over and over again, the following mixture is recommended: Cop¬ per, 90 parts; tin, 8 parts ; 5% phosphor tin, 2 parts. The scrap from this mix¬ ture may be used over and over again, with good results. 4. —Phosphor bronze, for use as bear¬ ings, which is one of the principal uses [87]](https://iiif.wellcomecollection.org/image/b31361523_0101.jp2/full/800%2C/0/default.jpg)