The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins.
- Albert A. Hopkins
- Date:
- 1910
Licence: Public Domain Mark
Credit: The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins. Source: Wellcome Collection.
112/1096 page 98
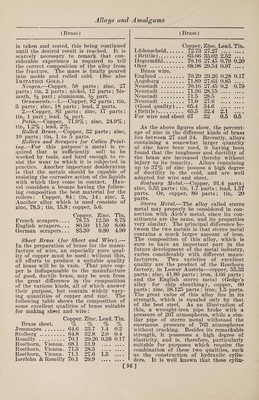
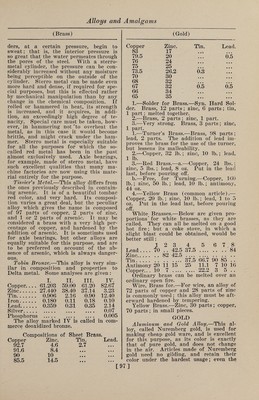
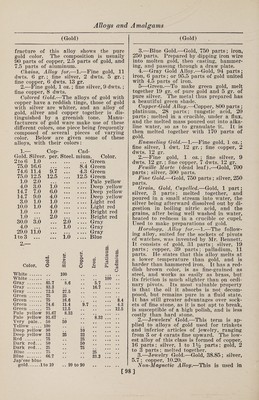
![(Gold) fracture of this alloy shows the pure gold color. The composition is usually 90 parts of copper, 2.5 parts of gold, and 7.5 parts of aluminum. Chains, Alloy for.—1.—Fine gold, 11 dwts. 6 gr.; fine silver, 2 dwts. 5 gr.; fine copper, 6 dwts. 13 gr. 2.—Fine gold, 1 oz.; fine silver, 9 dwts.; fine copper, 8 dwts. Colored Gold.—The alloys of gold with copper have a reddish tinge, those of gold with silver are whiter, and an alloy of gold, silver and copper together is dis¬ tinguished by a greenish tone. Manu¬ facturers of gold ware make use of these different colors, one piece being frequently composed of several pieces of varying color. Below are given some of these alloys, with their colors: 1.— Cop- Cad- Gold. Silver, per. Steel, mium. Color. 2 to 6 1.0 • • • • • • ... Green 75.0 16.6 • • • • ♦ • 8.4 Green 74.6 11.4 9.7 • • • 4.3 Green 75.0 12.5 12.5 • • • 12.5 Green 1.0 2.0 • • • • • • .... Pale yellow 4.0 3.0 1.0 • • • .... Deep yellow 14.7 7.0 6.0 • • • .... Deep yellow 14.7 9.0 4.0 • • • .... Deep yellow 3.0 1.0 1.0 • • • .... Light red 10.0 1.0 4.0 • • • .... Light red 1.0 ... 1.0 • • • .... Bright red 1.0 ... 2.0 • • • .... Bright red 30.0 3.0 • • • 2.0 .... Gray 4.0 ... • • • 1.0 .... Gray 29.0 11.0 • • • • • • .... Gray lto 3 ... • • • 1.0 .... Blue 2.— a • tl Color. Gold. Silvei Coppe Iron. Platii admiu o White. # # 100 • • •• • • • •• White . 100 .. Gray . 85.7 8.6 5.7 . Gray . 83.3 , . 16.7 . Gray . 72.5 27.5 Green . 75 25 Green . 75 16.6 . 8.4 Green . 74.6 11.4 9.7. 4.3 Green . 75 12.5 .12.5 Pale yellow 91.67 8.33 Pale yellow 91.67 . # 8.33. Very pale.. 50 50 Yellow . 100 Deep yellow 90 io . Deep yellow 53 25 22 . Red . 75 a • 25 . Dark red... 50 • • 50 . Dark red... 25 9 • 75 . Blue . 75 25 . Blue -... Jap’ese blue 66.7 • • 33.3 . gold.lto 10 .. 99 to 90 . (Gold) 3. —Blue Gold.—Gold, 750 parts ; iron, 250 parts. Prepared by dipping iron wire into molten gold, then casting, hammer¬ ing, and passing through a draw plate. 4. —Gray Gold Alloy.—Gold, 94 parts ; iron, 6 parts ; or 95.5 parts of gold united with 4.5 parts of iron. 5—Green.—-To make green gold, melt together 19 gr. of pure gold and 5 gr. of pure silver. The metal thus prepared has a beautiful green shade. Copper-Gold Alloy.—Copper, 800 parts ; platinum, 28 parts; tungstic acid, 20 parts ; melted in a crucible, under a flux, and the melted mass poured out into alka¬ line water, so as to granulate 'it. It is then melted together with 170 parts of gold. Enameling Gold.—1.—Fine gold, 1 oz.; fine silver, 1 awt. 12 gr.; fine copper, 2 dwts. 12 gr. 2.—Fine gold, 1 oz.; fine silver, 9 dwts. 12 gr.; fine copper, 7 dwts. 12 gr. Feuille Morte (dead leaf).—Gold, 700 parts ; silver, 300 parts. Fine Gold.—Gold, 750 parts ; silver, 250 parts. Grain, Gold, Cupelled.—Gold, 1 part; silver, 3 parts; melted together, and poured in a small stream into water, the silver being afterward dissolved out by di¬ gestion in boiling nitric acid, and the grains, after being well washed in water, heated to redness in a crucible or cupel. Used to make preparations of gold. Horology, Alloy for.—1.—The follow¬ ing alloy, suited for the sockets of pivots of watches, was invented by Mr. Bennett. It consists of gold, 31 parts; silver, 19 parts; copper, 39 parts; palladium, 11 parts. He states that this alloy melts at a lower temperature than gold, and is harder than hammered iron. It has a red¬ dish brown color, is as fine-grained as steel, and works as easily as brass, but its friction is much slighter than pn ordi¬ nary pivots. Its most valuable property is that the oil it absorbs is not decom¬ posed, but remains pure in a fluid state. It has still greater advantages over sock¬ ets of fine stone, as it is not apt to break, is susceptible of a high polish, and is less costly than hard stone. 2. —Jewelers’ Gold.—This term is ap¬ plied to alloys of gold used for trinkets and inferior articles of jewelry, ranging from 3 or 4 carats fine upward. The low¬ est alloy of this class is formed of copper, 16 parts ; silver, 1 to 1% parts; gold, 2 to 3 parts ; melted together. 3. —Jewelry Gold.—Gold, 38.85 ; silver, 5.7; copper, 10.20. Non-Magnetic Alloy.—This is used in ]](https://iiif.wellcomecollection.org/image/b31361523_0112.jp2/full/800%2C/0/default.jpg)