The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins.
- Albert A. Hopkins
- Date:
- 1910
Licence: Public Domain Mark
Credit: The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins. Source: Wellcome Collection.
113/1096 page 99
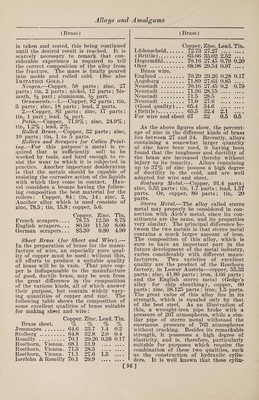
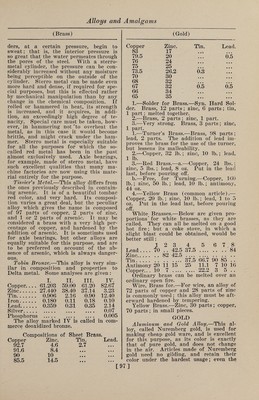
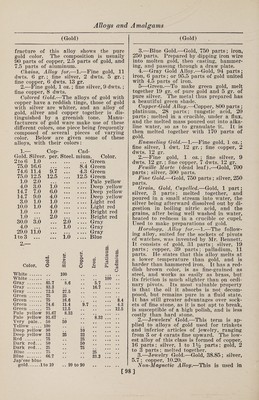
![(Gold) some of the Swiss watches to take the place of steel in the hair springs. It is composed of equal parts of gold and pal¬ ladium, copper about 15% of the whole, and a trace of rhodium and manganese are added; this may vary from l-10th of 1% to 5% of each. The copper and manganese are first added. Nurnberg Gold.— (See Aluminum and Gold.) Palladium.—1.—An alloy of palla¬ dium 20 parts, gold 80, is white, hard as steel, unchangeable in the air, and can, like the other alloys of palladium, be used for dental purposes. 2.—Alloys of gold, copper, silver, and palladium have a brownish red color and are as hard as iron. They are some¬ times (although rarely) used for the bearings for the pivots of the wheels of fine watches, as they cause less friction than the jewels commonly used for the purpose, and do not rust in the air. The composition used in the Swiss and Eng¬ lish watch factories consists usually of gold 18 parts, copper 13, silver 11, and palladium 6. Red Gold.—Gold, 750 parts; copper, 250 parts. Ring Gold.—Coin gold, 49.6 parts; sil¬ ver, 12.3 parts ; refined copper, 23.6 parts. Shakdo.—This is a famous Japanese alloy. It is composed of copper and gold, the proportions of the latter being va¬ riable, being from 2 to 8%. Talmi Gold.—The name of talmi gold was first applied to articles of jewelry, chains, earrings, bracelets, etc., brought from Paris, and distinguished by beauti¬ ful workmanship, a low price, and great durability. Later, when this alloy had acquired a considerable reputation, arti¬ cles were introduced under the same name, but really made of other metals, and wThich retained their beautiful gold color only as long as they were not used. The fine varieties of talmi gold are man¬ ufactured from brass, copper, or tombac, covered with a thin plate of gold, com¬ bined with the base by rolling, under strong pressure. The plates are then rolled out by passing through rollers and the coating not only acquires considerable density, but adheres so closely to the base that the metal will keep its beautiful ap¬ pearance for years. Of late, many arti¬ cles of talmi gold are brought into the market whose gold coating is produced by electroplating and is in many cases so thin that hard rubbing will bring through the color of the base. Such articles, of course, are not durable. In genuine talmi gold, the coating, even though it may be (Gold Imitations) thin, adheres very closely to the base, for the reason that the two metals are actually joined by the rolling, and also because alloyed gold is always used, which is much harder than pure gold. The pure gold of electroplating is very soft. The composition of some varieties of talmi gold are here given. It will be seen that the content of gold varies greatly, and the durability of the alloy will, of course, correspond to this. The alloys I, II and III are genuine Paris talmi gold; IV, V and VI are electroplated imitations; and VII is an alloy of a wrong composition, to which the gold does not adhere firmly : I. II. III. IV. v. vi. VII. Copper. 89.88 90.79 90.00 90.69 87.48 93.46 86.4 Copper. 88.16 83.13 84.55 .... Zinc. 9.32 8.33 8.9 88.97 12.44 6.60 12.2 Zinc.H.42 16.97 15.79 Tin. ii Iron.” 0^3 Gold. 1.03 0.97 0.91 0.5 0.3 0.05 .... White Gold, Electrum.—Gold whitened by addition of silver. Yellow Gold, Antique.—Pure gold. Imitation Gold Alloys. 1-—Gold Dutch, Mannheim gold, mosaic gold, ormolu, pinchbeck, Prince’s metal, red brass similor, tombac. These names are applied to several varieties of fine gold-colored brass, differing slightly in tint, and in the proportions of copper and zinc. At the celebrated works of Heger- miihl, near Potsdam, the proportions, cop¬ per 11 parts to zinc 2 parts, are employed to produce a metal which is afterward rolled into sheets for the purpose of mak¬ ing Dutch leaf gold. This alloy has a very rich, deep gold color. Its malleabil¬ ity is so remarkable that it may be beaten out into leaves not exceeding 1-52900 inch in thickness. 2.—The imitation gold alloys of differ¬ ent shades of yellow, dark, pale, or green¬ ish, are extensively used for cheap gold- colored coatings. The principal places where this special industry is carried on are Vienna, Nuremberg, and Furth, and it is usually pursued in connection with the manufacture of bronze powder. The composition of these alloys varies from 77 to 85 parts of copper and 23 to 15 parts of zinc. The metals are melted in graphite cru¬ cibles, and kept fluid for some time, in order that the alloy may become perfectly uniform. It is then cast into semi-circu¬ lar ingots about 23% inches long and % of an inch in diameter. These ingots are rolled cold into strips about the thick¬ ness of ordinary writing paper. Each strip is folded together so as to form a [99]](https://iiif.wellcomecollection.org/image/b31361523_0113.jp2/full/800%2C/0/default.jpg)