The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins.
- Albert A. Hopkins
- Date:
- 1910
Licence: Public Domain Mark
Credit: The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins. Source: Wellcome Collection.
121/1096 page 107
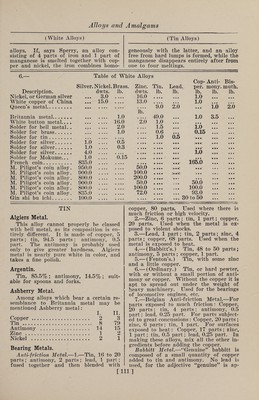
![(Silver Alloys) inum unite to form a pale yellowish-white alloy, perfectly malleable, susceptible of a high polish, equal to copper in fusibility and to nickel in magnetic power. Platinum and Silver.—An addition of platinum to silver makes it harder, but also more brittle, and changes the white color to gray; an alloy which contains only a very small percentage of platinum is noticeably darker in color than pure silver. Such alloys are prepared under the name of “platine au titrecontaining between 17 and 35% of platinum. They are almost exclusively employed for dental purposes. Watch Manufacturers, Alloys for.— Some very tenacious and hard alloys for making the parts of watches which are not sensitive to magnetism are as fol¬ lows : I. II. III. IV. V. VI. VII. Platinum.... 62.75 62.75 62.75 54.32 0.5 0.5.... Copper. 18.00 16.20 16.20 16.00 18.5 18.5 25.0 Nickel. 18.00 18.00 16.50 24.70.... 2.0 1.0 Cadmium.... 1.25 1.25 1.25 1.25 . Cobalt. 1.50 1.96 .... .. Tungsten. 1.80 1.80 1.77 . Palladium. 72.0 72.0 70.0 Silver. 6.5 7.0 4.0 Rhodium. 1.0. SILVER Silver and Aluminum.—1.—Alloys of these metals were made some years ago, and it was thought that valuable metals of a white color, and unaffected by the at¬ mosphere, would be obtained, which would make them superior to ordinary silver- copper alloys; but these great expecta¬ tions have not as yet been realized. Alum¬ inum hardens silver, and the alloys admit of a high polish. 2.—Tiers-Argent.—This alloy is chiefly prepared in Paris, and used for the man¬ ufacture of various utensils. As indi¬ cated by its name (one-third silver), it consists of 33.33 parts of silver and 66.66 parts of aluminum. Its advantages over silver consist in its lower price and great¬ er hardness; it can also be stamped and engraved more easily than the alloys of copper and silver. Silver and Antimony.—Alloys of these metals may be obtained in all propor¬ tions by direct fusion. They are hard, brittle, and gray or white in color. The whiteness decreases with the proportion of antimony. The alloys are very fusible, and wholly decomposed by cupellation or by fusion with niter, pure silver remain¬ ing. Mr. R. Smith has prepared the fol¬ lowing alloys: (Silver Alloys) I. II. III. Silver . 72.65 77.98 84.16 Antimony. 27.35 22.02 15.84 corresponding to the formulae 3Ag + Sb, 4Ag + Sb, and 6Ag + Sb, respectively. The silver was melted first, under a layer of charcoal, and the antimony then added. No. I was hard, crystalline, and bluish white; No. II was similar to No. I, but grayish white ; No. Ill was hard, granu¬ lar, and grayish white. The specific grav¬ ities of 48 silver-antimony alloys contain¬ ing 50% of silver and upward, have been determined by Cooke, of Harvard, who found that the densities were above the mean densities of the constituents, the maximum being reached in the alloy con¬ taining 26.6% of antimony. Cooke also found that the crystallization of the al¬ loys becomes marked as the same compo¬ sition is approached. Silver and Arsenic.—These metals are capable of uniting in several proportions, forming hard, gray, brittle, and readily fusible alloys. Gehlen produced an alloy containing 16% of arsenic, which is com¬ pact, brittle, steel-gray, and fine-grained. Berthier describes an alloy of 14.8% of arsenic as dull gray, brittle, and crystal¬ line ; by burnishing, it acquires the luster and color of silver; it is very fusible, and not decomposed on heating. Guettier de¬ scribes an alloy containing 14% of ar¬ senic, formerly used for table ware. Mr. R. Smith prepared a hard and brittle, though somewhat tough alloy, which be¬ came white and lustrous on burnishing. It contained 18.54% of arsenic, and cor¬ responded to the formula Ag3As. Silver- arsenic alloys may be prepared by direct fusion of the constituent metals, or by melting a mixture of silver, arsenious acid and black flux. Alloys containing small quantities of arsenic were formerly used in England in the manufacture of table ware. They are not, however, suitable for this purpose, on account of the poisonous character of the arsenic. They are com¬ posed usually of 49 parts of silver, 49 parts of copper and 2 parts of arsenic. Silver and Bismuth.—Alloys of these metals are hard, easily fusible, brittle, and lamellar in structure. The color of the 50% silver alloy is the same as that of bismuth. An alloy containing 33.33% of silver is said to be steel gray and to expand on solidification. Schneider states that when impure bismuth, containing sulphur, arsenic, iron, nickel and silver, is fused, and poured upon a cold plate, the globules of metal which are thrown up during solidification of the mass con- [107]](https://iiif.wellcomecollection.org/image/b31361523_0121.jp2/full/800%2C/0/default.jpg)