The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins.
- Albert A. Hopkins
- Date:
- 1910
Licence: Public Domain Mark
Credit: The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins. Source: Wellcome Collection.
124/1096 page 110
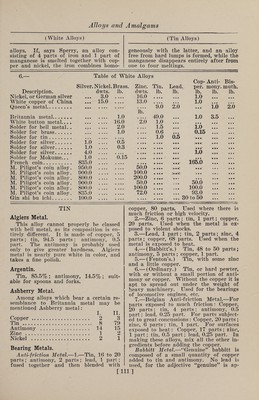
![(Silver Alloys) (Silver Substitutes) mass is thoroughly stirred with an iron rod, and at once poured out into molds. Alloys of silver and zinc can be obtained which are both ductile and flexible. An alloy consisting of 2 parts of zinc and 1 part of silver closely resembles silver in color, and is quite ductile. With a larger proportion of zinc the alloys become brit¬ tle. In preparing the alloy, a somewhat larger quantity of zinc must be taken than the finished alloy is intended to con¬ tain, as a small amount always volatil¬ izes. Berthier prepared an alloy contain¬ ing 80% of silver, which he states was rolled into very thin leaf; it was rigid, elastic, very tenacious, and tough. Mr. G. H. Godfrey prepared the following al¬ loys by pouring molten zinc into molten silver: -c — I. II. III. IV. Silver.. . 8.16 22.47 49.72 67.58 Zinc... . 91.84 77.53 50.28 32.42 I. The surface was bluish gray. The metal was hard, easily frangible, and easily scratched with a knife. Its frac¬ ture was bluish gray, finely granular, and feebly lustrous. II. The surface was bluish gray. The metal was harder than No. I, easily fran¬ gible, but less easily scratched. Its frac¬ ture was bluish gray, bright, and fibro- columnar. III. The surface was copper red after solidification. The metal was hard, brit¬ tle, and easily pulverized. The broken surface, when fractured cold, was white and very bright, and somewhat columnar. IV. The surface had a faint reddish- yellow tint. The metal was hard and easily frangible; its fracture white and very bright, but it soon tarnished; it was columnar in structure. An alloy of 2 parts by weight of zinc and 1 part of silver is said to be ductile, finely granular, and nearly as white as silver. Silver-zinc alloys have been proposed for coinage purposes. Piligot prepared alloys containing 5, 10 and 20% of zinc, respectively. They were white, with a tinge of yellow. The coins were elastic and sonorous. These alloys are not so readily blackened by sulphuretted hydro¬ gen as silver-copper alloys. Silver Substitutes.—1.—A writer gives the constituents of a hard alloy which lias been found very useful for the spac¬ ing levers of typewriters. The metal now generally used for this purpose by the various typewriter companies is “alumi¬ num. silver,” or “silver metal.” The pro¬ portions are given as follows: Copper, 57% ; nickel, 20% ; zinc, 20% ; aluminum, 3%. This alloy, when used on typewrit¬ ing machines, is nickel-plated, for the sake of the first appearance ; but so far as corrosion is concerned, nickeling is un¬ necessary. In regard to its other quali¬ ties, they are of a character that rec¬ ommends the alloy for many purposes. It is stiff and strong, and cannot be bent to any extent without breaking, especially if the percentage of aluminum is in¬ creased to 3.5% ; it casts free from pin¬ holes and blowholes. The liquid metal completely fills the mold, giving sharp, clean castings, true to pattern; its cost is not greater than brass; its color is silver white, and its hardness makes it susceptible of a high polish. 2. —Iron, 65 parts; tungsten, 4 parts; melted together and granulated. Also nickel, 23 parts ; aluminum, 5 parts; cop¬ per, 5 parts; in a separate crucible, to which is added a piece of sodium, in order to prevent oxidation. The two granulated alloys are then melted togeth¬ er. Both alloys resist the action of sul¬ phuretted hydrogen. 3. —Copper, 71 oz.; zinc, 7 oz.; nickel, 16% oz. ; iron, 1% oz.; cobalt (oxide), 1% oz.; tin, 2% oz. First fuse the zinc with 12 parts of the copper; then fuse the nickel with its own weight of the zinc alloy in a good black-lead crucible, and the iron, the remainder of the cop¬ per, and the oxide of cobalt mixed with charcoal. Cover the mass with charcoal, lute, and expose to a high heat. When properly fused, allow the heat to subside, and add the remainder of the copper-zinc alloy when the temperature is just suffi¬ cient to fuse it. Remove the crucible from the fire, and stir its contents well with a hazel stick. Wrap the tin in sev¬ eral thicknesses of dry paper, drop it into the alloy, stir for a moment, and run into the molds. When cold it is ready to be wrought like silver, which it resem¬ bles in every respect. The zinc is nearly all volatilized during the process of fusion. 4. —Aluminum Silver.—The following alloy takes a high silver polish, and ex¬ hibits a beautiful silver color: Copper, 70 parts ; nickel, 23 parts; aluminum, 7 parts. 5. —Sterlin.—A white metal resembling silver has found its way on the market under the name of sterlin, which has been found to contain 68.52% of copper, 12.84% of zinc, 17.88% of nickel, 0.76% of iron, and traces of lead. Silver and manganese were absent. Manganese is very useful for introducing iron into such [110]](https://iiif.wellcomecollection.org/image/b31361523_0124.jp2/full/800%2C/0/default.jpg)