The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins.
- Albert A. Hopkins
- Date:
- 1910
Licence: Public Domain Mark
Credit: The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins. Source: Wellcome Collection.
132/1096 page 118
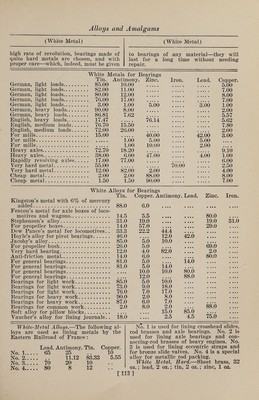
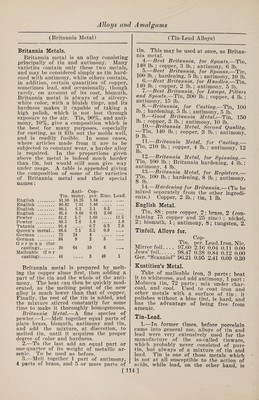
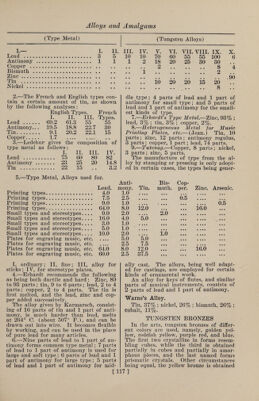
![(Zinc Alloys) from mixtures poor in acid, the other two from those containing more acid. But the color is dependent not merely on the composition of the soda tungstate salt, but also on the amount of tin, and on the duration of the fusion; so that when much tin is used, and the fusion is pro¬ longed, a yellow bronze is obtained from a very acid mixture, and, on the contrary, a salt that is but slightly acid, when fused only a short time and with very lit¬ tle tin, may yield a red or even a blue bronze. A mixture in the proportion of 2 mole¬ cules of soda tungstate and 1 molecule of anhydrous tungstic acid, with tinfoil slow¬ ly added, and kept melted for 1 or 2 hours, will yield cubes 1-5 in. long when about 4 oz. are melted, and they will pro¬ duce a yellow or reddish-yellow bronze, the powder of which seems light brown, and when stirred up with water it im¬ parts to the liquid the property of ap¬ pearing of a fine blue color by transmit¬ ted light. The red bronze obtained from 10 parts of soda carbonate, 70 parts of soda tung¬ state, and 20 parts of tinfoil, yields, on pulverization, a powder that, stirred up in water, transmits green light. According to J. Philipp, a blue bronze is always obtained, if the fused mixture contains more than 3 molecules of tung¬ stic acid to 1 molecule of soda; if the fused product is boiled alternately with muriatic acid and with carbonate of soda, the result will be a considerable quantity of fine blue prismatic crystals, with which there are intermixed, in most cases, sin¬ gle red and yellow cubes. Moreover, all the tungsten bronzes obtained by fusion with tin can also be prepared by electroly¬ sis of fused acid tungstates, but the yield is so small that it is unprofitable. ZING Bidery, Vidry.—1.—An alloy of which the chief seat of manufacture is the city of Bider, near Hyderabad, India. Many articles made of it were greatly admired at the International Exhibition of 1851. Its color is between that of pewter and zinc, does not corrode by exposure to air or damp, and can only be broken by ex¬ treme violence. Zinc, 31 parts; copper and lead, each 2 parts ; melted together, with the usual precautions, under a mix¬ ture of rosin and beeswax, to prevent oxidation. 2.— (Dr. Heyne.) Copper, 8 parts; lead, 2 parts ; tin, 1 part; melted as be¬ fore. For use, the resulting alloy is re¬ (Zinc Alloys) melted, and to every 3 parts of it 16 parts of zinc are added. 3.—Genuine Indian Bidery metal (fre¬ quently imitated in England) is about as follows : „ % % Copper. 3.5 11.4 Zinc . 93.4 84.3 Tin ... 1.4 Lead. 3.1 2.9 Zinc Bronzes (Fontaine Moreau). Zn. Cu. Fe. Pb. . 90 8 1 1 91 8 0 1 92 8 0 0 92 7 1 0 The above may be considered the maxi¬ mum of zinc and minimum of copper that will cast free of crystalline fracture. By lessening the zinc from 1 to 4%, and in¬ creasing the copper 1-8 to 1-6, a better texture may be looked for. Zinc-Nickel.—Zinc, 9 parts ; nickel, 1 part. Used for painting. BoreVs Alloy.—This alloy has conspic¬ uously valuable properties, which adapt it to many purposes. Its most striking characteristic is its hardness, which equals that of good wrought iron, while in tenac¬ ity it surpasses the best cast iron. In casting, it is readily detached from the mold, and can be mechanically worked with great ease, but is too brittle to be rolled out into sheets or drawn into wire. It takes all the lines of the mold exceed¬ ingly well, and on this account is much used for casting small statues, which, aft¬ er careful bronzing, are given the name of cast bronze. The large proportion of zinc contained in this alloy makes the price of production comparatively low. It is quite suitable for the manufacture of articles which are to be exposed to the influences of the weather, as it does not easily rust, and becomes covered, after a while, with a thin layer of oxide, which prevents further oxidation. Two mix¬ tures, given below, have practically the same properties, although they vary con¬ siderably in actual composition. I. II. Copper. 1 10 Zinc. 98 80 Iron . 1 10 The iron is used in the form of cast- iron shavings, added to the zinc. The copper is then added, and the alloy kept fluid for some time, under cover of glow¬ ing coals, in order to insure an intimate combination of the metals, without the combustion of the zinc. The combusti- [118]](https://iiif.wellcomecollection.org/image/b31361523_0132.jp2/full/800%2C/0/default.jpg)