The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins.
- Albert A. Hopkins
- Date:
- 1910
Licence: Public Domain Mark
Credit: The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins. Source: Wellcome Collection.
55/1096 page 41
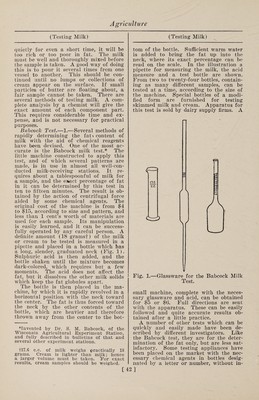
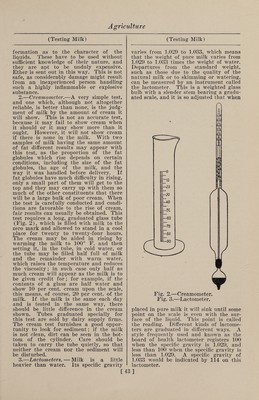
![covered tin pail answers well for the larger vessel. An inverted pie pan with perforated bottom can serve as the false bottom. A hole may be punched in the cover of the pail, a cork inserted, and a chemical thermometer put through the cork so that the bulb dips in the water, thus enabling one to watch the tempera¬ ture closely without removing the cover, or an ordinary dairy thermometer may be used from time to time by removing the lid. Preservation of Milk. 1. —A mixture of 2 drams boracic acid with 3 drams common salt, of which an addition of 2-3 dram to 1 gal. of milk is said to increase its keeping qualities for twenty-four hours. 2. —When milk contained in wire- corked bottles is heated to the boiling point in a water bath, the oxygen of the included small portion of air under the cork seems to be carbonated, and the milk will, it is said, keep fresh for a year or two. 3. —A small quantity of boracic acid added to milk will keep it from souring and delay the separation of cream. It can be kept several days by this means. 4. —Fresh milk in bottles has been treated with oxygen and carbonic acid under pressure of some atmospheres. By this method it is said to be possible to preserve milk 50 to 60 days in a fresh state. The construction of the bottles is siphon-like. A bacteriological examina¬ tion of the preserved milk is still out. 5. —Engineer Budde, of Copenhagen, has discovered a preserving agent for milk which consists in adding to the milk, which should be as fresh as possible, enough hydrogen peroxide to cause it to be completely decomposed by the enzymes of the milk. For this purpose 1.3 per cent., by volume, of a 3 per cent, hydro¬ gen peroxide solution is required. The milk is well shaken and kept for five hours at 50 to 52° C. in well-closed ves¬ sels. Upon cooling, it is said to keep fresh for about a month and also retain its natural fresh taste. With this process, if pure milk is used, the ordinary disease germs, it is claimed, are killed off soon after milking and the milk sterilized. For still longer conservation Budde adds an¬ other harmless preserving agent which he keeps secret, as it has not yet been pat¬ ented. 6. —Qlacialine.—According to Dr. Be- sana, this substance, which has met with so much favor in England and elsewhere as an antiseptic, especially for the preser¬ vation of milk, has the following com¬ position : Boracic acid, 18 parts; borax, 9 parts; sugar, 9 parts; glycerine, 6 parts. 7.—M or fit’s Process.—In 1 gal. milk at 130 to 140° F. (55 to 60° C.) is dissolved 1 lb. gelatine ; the mixture is left to cool to a jelly, when it is cut into slices and dried. The compound is used to gelati¬ nize more milk, and this is repeated till the gelatine is in the proportion of 1 lb. to 10 gal. of milk. Testing Milk. The following directions for detecting impure milk, including the use of the creamometer, lactometer and the Babcock test, is taken from Farmers’ Bulletin 42, issued by the United States Department of Agriculture: By pure milk is meant the properly handled product of healthy, well-fed cows. To be legally regarded as pure, in most places, milk must contain at least a cer¬ tain amount of fat and other solids. It is a difficult thing to determine by the ap¬ pearance of milk whether it is pure or not, and even experienced dairymen are frequently unable to do this. It has a slightly yellowish white color, a very slight odor, if any, and should have a dis¬ tinctly sweet and pure taste. When al¬ lowed to stand quietly for several hours, cream should rise naturally, and if the separation is thoroughly effected the cream should form one-eighth to one-fifth of the total volume or bulk. No sedi¬ ment should appear in the bottom of the jar or vessel. When good milk is poured from a tumbler it should cling to the glass a little and not run off clean like water. Skimmed or watered milk is thin¬ ner than whole milk and of a lighter shade, being of a bluish-white color. The yellow shade of milk is chiefly due to its fat, but as this constituent is more yel¬ low in the milk of some cows than others the yellowest milk is not necessarily the richest, and it is unsafe to judge by the color alone; poor milk from some cows may be more highly colored than rich milk from others. Besides this, artificial colors are sometimes added by dishonest persons. When a quantity of milk is to be tested, the first and most important thing to be done is to obtain a fair sample—one that will represent the whole and show its average composition. If the sample is taken from near the top or bottom of a vessel of milk which has been standing [41]](https://iiif.wellcomecollection.org/image/b31361523_0055.jp2/full/800%2C/0/default.jpg)