The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins.
- Albert A. Hopkins
- Date:
- 1910
Licence: Public Domain Mark
Credit: The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins. Source: Wellcome Collection.
89/1096 page 75
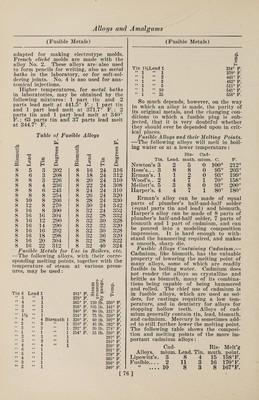
![(Bismuth and Cadmium) galvanized. When aluminized zinc is used, it has been found unnecessary to use sal ammoniac for clearing the bath of oxide, inasmuch as the aluminum ac¬ complishes the same purpose ; and if the two are used together they seem to coun¬ teract the effects of each other. Alumi¬ nized zinc should be added to the galva¬ nizing baths gradually as the bath is con¬ sumed, in quantities of about 1 lb. at a time for a 5-ton bath. This statement applies when a 5% aluminized zinc is used. The first action of aluminum in galvanizing baths is to make the bath more liquid, which is one of the objects in adding the aluminum. A great amount of aluminum seems to combine with the impurities in the zinc, and comes to the surface in the form of a scum, which makes galvanizing difficult. If, therefore, too much aluminum goes into the bath, stir the bath well, and allow it to stand for a while until the aluminum com¬ bines with these impurities and comes to the surface as a scum. Remove this scum, add some sal ammoniac to counter¬ act the effects of the aluminum, and re¬ duce the proportion of the aluminized zinc added. In starting with a new bath, it is especially important that these sug¬ gestions should be followed. BISMUTH AND CADMIUM ALLOYS Bismuth Bronze. 1.—A metallic alloy, which the in¬ ventor calls bismuth bronze, was intro¬ duced by Webster, as specially suitable for use in sea water, for telegraph and music wires, and for domestic articles. The composition varies slightly with the purpose for which the bronze is to be used, but in all cases the proportion of bismuth is very small. For a hard alloy he takes 1 part of bismuth and 16 parts of tin, and, having melted them, mixes them thoroughly as a separate or pre¬ liminary alloy. For a hard bismuth bronze he then takes 69 parts of copper, 21 parts of spelter, 9 parts of nickel, and 1 part of the bismuth-tin alloy. The met¬ als are melted in a furnace or crucible, thoroughly mixed, and run into molds for future use. This bronze is hard, tough, and sonorous ; it may be used in the manufacture of screw-propeller blades, shafts, tubes, and other appliances em¬ ployed partially or constantly in sea water, being especially suited to withstand the destructive action of salt water. In consequence of its toughness it is well suited for telegraph wires and other pur¬ poses where much strain has to be borne. (Fusible Metals) From its sonorous quality it is well adap¬ ted for piano and other music wires. For domestic utensils, and other articles gen¬ erally exposed to atmospheric influence, the composition is 1 part of bismuth, 1 part of aluminum, and 15 parts of tin, melted together to form the separate or preliminary alloy, which is added in the proportion of 1% to the above described alloy of copper, spelter and nickel. The resulting bronze forms a durable, bright and hard alloy, suited for the manufac¬ ture of spoons, forks, knives, dish covers, kettles, teapots, jugs, and numerous other utensils. These alloys are said to resist oxidation, to polish well and easily, and to keep their color well. I. II. III. IV. Copper . 25 45 69 47 Nickel . 24 32.5 10 30.9 Antimony. 50 Bismuth. 1 1 1 0.1 Tin. 16 15 1 Zinc. 21.5 20 21 Aluminum. . . 1 I is hard and very lustrous, suitable for lamp reflectors and axle bearings. II is hard, resonant, and not affected by sea water, for parts of ships, pipes, telegraph wires and piano strings; III and IV are for cups, spoons, etc. 3.—Tin, 16 parts; bismuth, 1 to 3 parts. Fusible Alloys. Under the name, fusible metal, or fus¬ ible alloy, is understood a mixture of metals which becomes liquid at tempera¬ tures at or below the boiling point of water. 1. —D’Arcet's.—Bismuth, 8 parts ; lead, 5 parts ; tin, 3 parts. This melts below 212° F. 2. —Walker's.—Bismuth, 8 parts : tin, 4 parts; lead, 5 parts; antimony, 1 part. The metals should be repeatedly melted and poured into drops until they can be well mixed, previous to fusing them to¬ gether. 3. —Onion's.—Lead, 3 parts ; tin, 2 parts; bismuth, 5 parts. Melts at 197° F. 4. —If to the latter, after removing it from the fire, 1 part of warm quicksilver be added, it will remain liquid at 170° F., and become a firm solid only at 140° F. 5. —Bismuth, 2 parts ; lead, 5 parts; tin, 3 parts. Melts in boiling water. Nos. 1, 2, 3 and 5 are used to make toy spoons to surprise children by their melting in hot liquors. A little mercury (as in 4) may be added to lower their melting points. Nos. 1 and 2 are specially [75]](https://iiif.wellcomecollection.org/image/b31361523_0089.jp2/full/800%2C/0/default.jpg)