The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins.
- Albert A. Hopkins
- Date:
- 1910
Licence: Public Domain Mark
Credit: The Scientific American cyclopedia of formulas : partly based upon the twenty-eighth edition of Scientific American cyclopedia of receipts, notes and queries 15,000 formulas / edited by Albert A. Hopkins. Source: Wellcome Collection.
96/1096 page 82
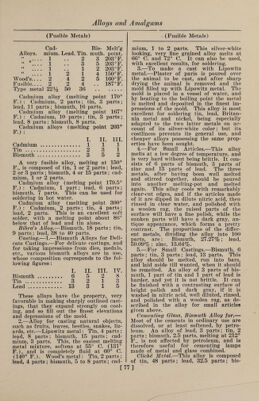
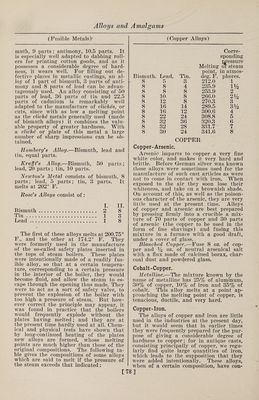
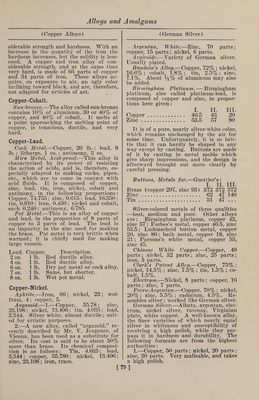
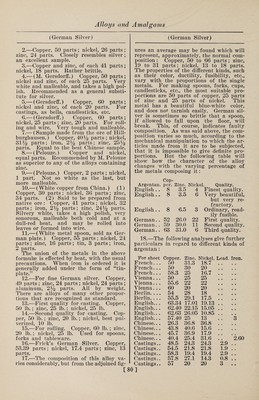
![(Bell Metal) Toned1 s Metal.—Copper, 5 parts ; nickel, 4 parts; tin, 1 part; lead, 1 part; iron, 1 part; zinc, 1 part; antimony, 1 part. It is hard, difficult to fuse, not very duc¬ tile, and cannot be recommended. Copper-Phosphor. Phosphor copper may be prepared in a variety of ways: (1) By dropping phos¬ phorus upon molten copper in a crucible, an alloy rich in phosphorus is obtained, forming an extremely hard steel-gray fus¬ ible compound. (2) By reducing phos¬ phate of copper with charcoal, or char¬ coal and carbonate of soda. (3) By. heat¬ ing a mixture of 4 parts of bone ash, 1 part of charcoal and 2 parts of granu¬ lated copper at a moderate temperature. The melted phosphide of copper separates on the bottom of the crucible, and is stated to contain 14% of phosphorus. (4) By adding phosphorus to copper-sulphate solution and boiling. The precipitate is dried, melted, and cast into ingots. When of good quality, and in proper condition, it is quite black. (5) Copper phosphide is easily prepared by adding to a crucible 14 parts of sand, 18 parts of bone ash, 4 parts of powdered coal, 4 parts of so¬ dium carbonate, and 4 parts of powdered glass ; the whole being intimately mixed with 9 parts of granulated copper. A lid is then luted on and the crucible exposed to a strong heat. The sand acts on the bone ash, forming silicate of lime. The liberated phosphoric acid is reduced by the coal, and the phosphorus thus set free unites with the copper. (6) Montefiori- Levi and Kunzel prepare phosphor copper by putting sticks of phosphorus into cru¬ cibles containing molten copper. To avoid a too ready combustion the sticks of phos¬ phorus are previously coated with a firm layer of copper, by placing them in a so¬ lution of copper sulphate. (7) By strong¬ ly heating in a crucible an intimate mix¬ ture of bone ash, copper oxide and char¬ coal, phosphor copper is produced. Copper-Tin. Bell Metal.—1.—The various alloys used in the manufacture of bells consist essentially of copper and tin, but in some cases other metals are added in small quantities, either for cheapness or to pro¬ duce a desired quality of sound. The ad¬ ditional metals chiefly used are zinc, lead, iron, and sometimes bismuth, silver, anti¬ mony and manganese. The following are some of the proportions employed: Musi¬ cal bells, 84% copper, 16% tin. Sleigh bells, 84.5% copper, 15.4% tin, 0.1% an¬ timony. Gongs, 82% copper, 18% tin. (Bell Metal) House bells, 80% copper, 20% tin. House bells, 78% copper, 22% tin. Large bells, 76% copper, 24% tin. Swiss clock bells, 74.5% copper, 25% tin, 0.5% lead. Old bell at Rouen, 71% copper, 26% tin, 1.8% zinc, 1.2% lead. Clock bells, 72% copper, 26.56% tin, 1.44% silver. Alarm bell at Rouen, 75.1% copper, 22.3% tin, 1% zinc, 1.6% silver. Tam-tam, 79% copper, 20.3% tin, 0.52% lead, 0.18% silver. Japanese kara kane, 64% copper, 24% tin, 9% zinc, 3% iron. Japanese kara kane, 70% cop¬ per, 19% tin, 3% zinc, 8% lead. Japa¬ nese kara kane, 61% copper, 18% tin, 6% zinc, 12% lead, 3% iron. White table bells, 17% copper, 80% tin, 3% bismuth. White table bells, 87.5% tin, 12.5% anti¬ mony. Small bells, 40% copper, 60% tin. 2. —The composition of bell metal can be varied considerably, and the tone of the bell varies accordingly, as may be seen from the following: Normal com¬ position, 80% copper, 20% tin. Normal composition, 78% copper, 22% tin. Rouen alarm bell, 76.1% copper, 22.3% tin, 1.6% zinc, 1.6% silver. Ziegenhain alarm bell, 71.48% copper, 33.59% tin, 4.04% lead, 0.12% iron. Darmstadt alarm bell, 73.94% copper, 21.67% tin, 1.19% lead, 0.17% silver. Reichenhall alarm bell (13th century), 80% copper, 20% tin. Tam-tam, 78.51% copper, 10.27% tin, 0.52% lead, 0.18% silver. Japanese bells, 1.10% copper, 4% tin, 1.5% zinc, 0.5% silver. Japanese bells, 2.10% copper, 2.5% tin, 0.5% zinc, 1.33% lead. Japa¬ nese bells, 3.10% copper, 3% tin, 1% zinc, 2% lead, 0.5% silver. Japanese bells, 4.10% copper. Small clock bells, table bells, sleigh bells, etc., require an alloy which will give a clear and pure tone. It has been learned by experience that bell metal containing about 22% of tin gives the highest tone, and is, there¬ fore, suited to small bells. It is an ob¬ ject, however, in this case, to produce the alloy as cheaply as possible by reducing the proportion of the copper, its most ex¬ pensive component. The following will show the composition of the alloys used for small bells: (1) House bells, 80% copper, 20% tin. (2) House bells, small¬ er, 75% copper, 25% tin. (3) German clock bells, 73% copper, 24.3% tin, 2.7% zinc. (4) Swiss clock bells, 74.5% cop¬ per, 25% tin, 0.5% lead. (5) Paris clock bells, 72% copper, 26.56% tin, 1.44% sil¬ ver. (6) Sleigh bells, 84.5% copper, 15.42% tin, 0.1% silver. The alloy num¬ bered (6) contains, in addition, 0.1% of antimony. 3. —Melt together, under powdered char¬ coal, 100 parts of pure copper with 20 [82]](https://iiif.wellcomecollection.org/image/b31361523_0096.jp2/full/800%2C/0/default.jpg)