The chemistry of essential oils and artificial perfumes / by Ernest J. Parry.
- Parry, Ernest J. (Ernest John)
- Date:
- 1908
Licence: In copyright
Credit: The chemistry of essential oils and artificial perfumes / by Ernest J. Parry. Source: Wellcome Collection.
Provider: This material has been provided by UCL Library Services. The original may be consulted at UCL (University College London)
105/594 (page 93)
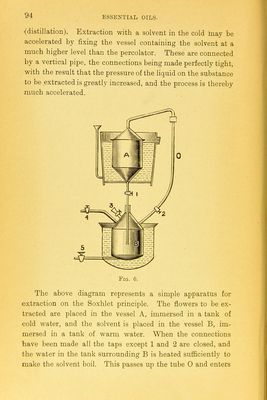
![The simplest process of extraction is merely an adaptation of the ordinary method of percolation, or, with a view to economise the solvent used, an apparatus on the principle of the ordinary Soxhlet tube is used. The chief point to be noted is the arrangement of the apparatus so that the minimum, quantity of the volatile solvent shall be lost. Various patents have been taken out in respect of extraction processes, amongst them being several involving the use of the very- volatile solvent methyl chloride. One of these is well illus- trated in the Pharmaceutical Journal [3], xiv., p. 44. Most, of these are very complicated as regards the arrangement of vessels and pumps, but a simple process, in which methyl chlo- ride is used, is described by Blogg in his evidence given before the Eoyal Commission of Inquiry into the vegetable products of Victoria. A jacketed vacuum still, fitted with the pump- and condenser, a closed macerating vessel and a receiver are all the apparatus necessary. The fresh flowers, for it is- nearly always fresh flowers that are treated in this way, are placed in the macerating vessel, which is filled up with pure methyl chloride and kept tightly fastened for a quarter of an hour. The liquid is then rapidly transferred to the vacuum pan, this is warmed and the exhaust pump is started.. The solvent is rapidly evaporated, leaving the extracted oil, etc., in the pan. Great care must be exercised to see that the methyl chloride is as pure as possible. Much of the commercial product is manufactured from the trimethylamine obtained by destructively distilling the residues obtained from crystallisation of beet sugar. To purify it, it is best to pass it through hydrochloric acid, and then to dry it with calcium chloride. It is then compressed into iron cylinders, when it forms a mobile liquid. To purify oils obtained by such processes it is necessary either to dissolve them out with alcohol from the wax, etc., which is extracted with them from the plants, or to purify them by a process of rectification](https://iiif.wellcomecollection.org/image/b21687596_0105.jp2/full/800%2C/0/default.jpg)