Licence: Public Domain Mark
Credit: The fever of over-exertion / by J.F. Knott. Source: Wellcome Collection.
Provider: This material has been provided by The Royal College of Surgeons of England. The original may be consulted at The Royal College of Surgeons of England.
10/36 (page 8)
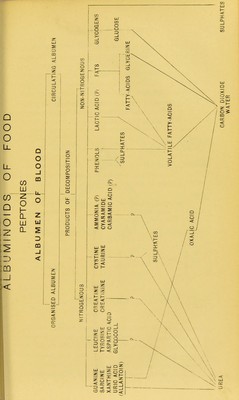
![The benzene nucleus which forms so prominent a feature in the latter, is well known to display the most tenacious resistance to chemical change. The albuminoid molecule, as a whole, is notably unstable, and the nature of the connection of the long chain of cyan-alcohol groups, offers an easy explanation of this fact. The detachment and mutual separation of the links of this long chain of molecular groups is necessarily accompanied by the evolution of a quantity of heat; and from the nature of so large a-molecular structure, as well as from the large proportion of the total mass of the tissues of the body formed by the muscular system, we can at •once understand the very important part played by the latter in producing the heat of the animal economy. In another diagram (see opposite page) I have arranged, in schematic form, the probable succession of the principal products •of proteid waste, beginning with their ingestion as albuminoids of food, and ending with their elimination from the system as effete matter. We have now an explanation of the presence of an excess of uric acid during the rapid and disorderly muscular metabolism of the febrile state. FATE OF THE DETACHED CYAN-ALCOHOL GROUPS. mi /OH ^CJl.^'C + 2H,0 = C„H,n:^ + NH3 ^CN ^CO.OH OH Corresponding acid of Acetic Series. •C„H2„:^ +02= Cn-,H,„_..CO.OH + CO2 + H2O. ^CO.OH Ethedene cyan-alcohol. Lactic acid. ^OH ,0H C 4. 2H,0 = C,H,:f + NH3 [? EHETBIATISIM]. ^CN ^CO.OH Lactic acid. OH Acetic acid. Formic acid. CHa.CH^' + 0 = CH3—CO.OH + H.CO.OH. ^CO.OH = CH3.CO.OH + 40 = 2H2O + 2C0, H.CO.OH + O = H2O 4- CO,;](https://iiif.wellcomecollection.org/image/b22279179_0010.jp2/full/800%2C/0/default.jpg)