Coloured analytical tables : showing the behaviour of the more common metals and acids to the ordinary reagents, with special reference to the colour of the various oxides, salts, precipitates, flames, borax-beads, and blowpipe reactions a class-book for students in hospitals, colleges and schools / by H. Wilson Hake.
- Hake, H. Wilson (Henry Wilson), 1857-1930
- Date:
- 1889
Licence: Public Domain Mark
Credit: Coloured analytical tables : showing the behaviour of the more common metals and acids to the ordinary reagents, with special reference to the colour of the various oxides, salts, precipitates, flames, borax-beads, and blowpipe reactions a class-book for students in hospitals, colleges and schools / by H. Wilson Hake. Source: Wellcome Collection.
18/40
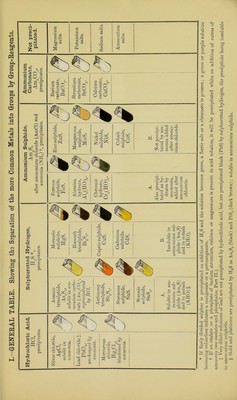
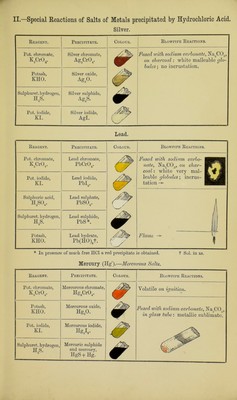
![Hydrogen (in the presence of Hydrochloric Acid). A. Metals whose Sulphides are soluble in Am2S or KHO. Arsenic. The reactions of arsenic in the General Table are sufficient for ordinary purposes, but the following reactions may be made. Marsh’s Test*.—This consists in the production of arseniuretted hydrogen, AsHa, by the addition of an arsenic compound in solution to an apparatus in which hydrogen is being generated. The AsH, so formed is a poisonous gas of garlic-like odour ; it burns with a pale blue flame, which deposits metallic arsenic in contact with a cold porcelain surface, and it is decomposed with deposition of metallic arsenic on heating a glass tube through which it is passing. Beinsch’s Test.—This consists in boiling small clean pieces of pure copper-foil in a hydrochloric acid solution of an As com- pound (strength of acid 1 : 6 water) for a few minutes, when, if As is present, it will be deposited as a grey coating on the copper foil; on drying and heating the foil (gently at first) in a thin perfectly dry tube closed at one end the deposit is oxidised to arsenious oxide, As20:j, which sublimes in octahedra recognizable with a low-power magnifying-glass. N.B.—This production of As203 crystals is also possible with the metallic deposit in Marsh’s test; absence of moisture is an essential condition. All arsenic compounds are volatile on ignition. Antimony. Reagent. Potash, KHO. Zinc and hydro- chloric acid, Zn and HC1. Precipitate. Antimony hydratet, Sb(HO)a. Sb. Colour. Sol. in xs. Black stain on platinum-foil. Blowpipe Reactions. Fused with sodium carbonate (Na2C03) on charcoal, white brittle glo- bules ; in- crustation. 0 t The solution of the hydrated oxide in hydrochloric acid (HOI) gives, with much water, a white precipi- tate of oxychloride of antimony (SbOCl), soluble in tartaric acid (H2C,H406) [distinction from Bismuth]. Tin. Stannous Salts. Reagent. Precipitate. Colour. Blowpipe Reactions. Potash, KHO. Stannous hvdrate, Sn(HO)2. Sol. in xs. Fused with sodium carbonate (Na2C03) on charcoal, white malleable globules; no incrustation. Gold chloride, AuClg. Purple of Cassius. In presence of a drop of chlo- rine-water. Mercuric chloride, HgCl2. Mercurous chloride and mercury, Hg2Cl2; Hg. & Grey on boiling. The stannous salt must be in xs. Zinc and hydro- chloric acid, Zn + HCl. Sn. /wju' Spongy mass. Stannic Salts. These also yield metallic tin with zinc and hydrochloric acid, and are reduced to stannous salts on addition of copper and long boiling. * Many precautions are necessary in making this test, which should be conducted in a draught cup- board, owing to the poisonous nature of the gas, and especial care should be taken to avoid lighting the issuing hydrogen before the whole of the air is driven out of the apparatus, or an explosion may easily occur. A beginner should not attempt it without skilled assistance.](https://iiif.wellcomecollection.org/image/b28109399_0018.jp2/full/800%2C/0/default.jpg)