Elementary quantitative analysis / by Alexander Classen ; translated, with additions, by Edgar F. Smith.
- Classen, Alexander, 1843-1934.
- Date:
- 1878
Licence: Public Domain Mark
Credit: Elementary quantitative analysis / by Alexander Classen ; translated, with additions, by Edgar F. Smith. Source: Wellcome Collection.
319/352
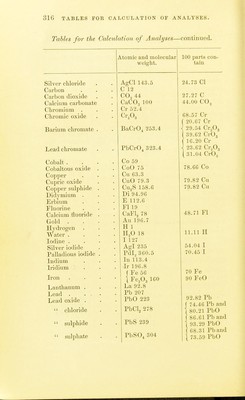
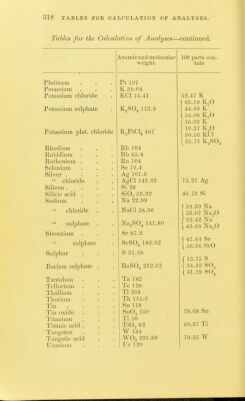
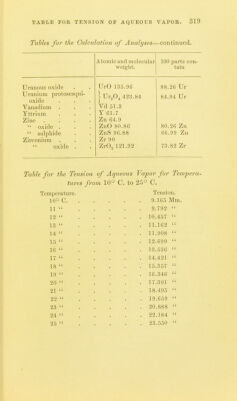
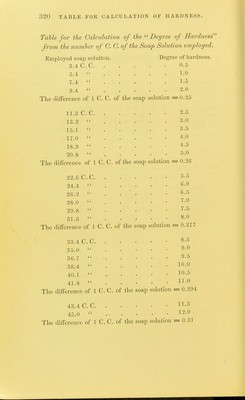
![TABLES FOR C ALC U L AT 1 () N OF ANALYSES. TAIU.es FOR THE C'AECULATION OF ANALYSES. Atomic und molecular 100 i)arts con- weight. tain Aliimimim . Al 27.3 ■ Aliuniiuiiu oxide . Al.Og 1()2.4,S 53.28 Al Aiitiiiiony Sb']22 Aiitiiuoniate of teroxide of antimony . SbO., li)4 79.22 Sb Antimony suljiliide Sb.,S3 340 71.76 Sb Arsenie As'74.!) Magnesium p} io-arsenate Mg.ASjO, 309.4 48.42 As Ammonium - magnesium 2MgO(NH,).,0 + ( 39.49 As < 60.O3 As^Os arsenate As.,05+H,0 379.34 [ 80.44 As^Oj Barium Ba 13G.8 Barium sulphate BaSO^ 232.02 f 58.81 Ba [ 6o.6 I BaO Bnrinin sllieo-fluoride BaSiFlg 279.4 1 48.96 Ba 1 54.67 BaO Beryllium Be 9.3 Bismuth Bi 210 Bismuth oxychloride BiOCl 261.5 80.30 Bi Boron .... B 11 Boracic acid . B,,03 70 31.42 B Boro-fluoride of potassium KBFl^ 126.1 1 8.73 B [ 27.75 B.^Oj Bromine 80 Silver bromide AgBr 188 42.55 Br Cadmium Cd 112 Cadmium oxide CdO 128 87.50 Cd Cadmium sulphide CdS 144 1 77.77 Cd 1 88.88 CdO Caisium Cs 133 Calcium La 40 Calcium oxide CaO 56 71.42 Ca j 40.00 Ca Calcium carbonate CaCOg 100 1 50.00 Ca,0 Calcium sulphate . CaSO^ 130 j 29.41 Ca t 41.17 CaO Cerium Ce 92.10 Ciilorine CI 35.5](https://iiif.wellcomecollection.org/image/b2149776x_0321.jp2/full/800%2C/0/default.jpg)