Volume 1
Watts' dictionary of chemistry / revised and entirely rewritten by H. Forster Morley and M.M. Pattison Muir ; assisted by eminent contributors.
- Date:
- 1888-1894
Licence: Public Domain Mark
Credit: Watts' dictionary of chemistry / revised and entirely rewritten by H. Forster Morley and M.M. Pattison Muir ; assisted by eminent contributors. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
767/796 (page 741)
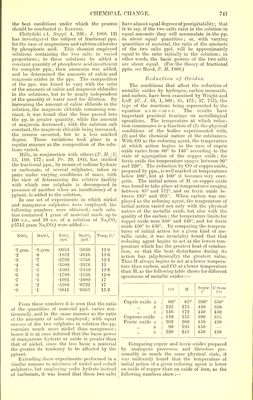
![the best conditions under which the process should be conducted (v. Earths). Chizyriski (A. Suppl. 4, 226; J. 1866. 12) has investigated the subject of fractional ppn. for the case of magnesium and calcium chlorides by phosphoric acid. This chemist employed solutions containing the two salts in varied proportions; to these solutions he added a constant quantity of phosphoric acid insufficient for complete ppn., then ammonia was added, and he determined the amounts of calcic and magnesic oxides in the pps. The composition of the pps. was found to vary with the ratio of the amounts of calcic and magnesic chlorides in the solutions, but to be nearly independent of the quantity of water used for dilution. By increasing the amount of calcic chloride in the solution, the magnesic chloride remaining con- stant, it was found that the lime passed into the pp. in greater quantity, while the amount of magnesia decreased; with the calcium salt constant, the magnesic chloride being increased, the reverse occurred, but to a less marked degree. These variations took place in a regular manner as the composition of the solu- tions varied. Mills, in conjunction with others (P. M. [5] 13, 169, 177; and Pr. 29, 181), has studied the fractional ppn., by means of sodium hydrate or carbonate, of several sulphates, taken in pairs under varying conditions of mass, with the view of determining the relative facility with which one sulphate is decomposed in presence of another when an insufficiency of a pptant. is added to the solution. In one set of experiments in which nickel and manganese sulphates were employed, the following numbers were obtained; each solu- tion contained 1 gram of material made up to 100 c.c., and 10 e.c. of a solution of NaoC03 (•5715 gram Na„CO;1) were added:— NiSO, MnSO„ NiSO„ ppd. MnSd ppd. Temp. C.° •1 grm. •9 grm. •0953 •5850 12-9 •2 ■8 •1852 •4616 13-6 •3 •7 •2799 •3766 12-5 •4 -6 •3588 •2976 13 •5 ■5 •4305 •2450 13-6 •6 •4 •4788 •1536 12-8 •7 •3 •4991 •1089 17 •8 •2 •5584 ■0722 17 •9 •1 •5841 •0363 152 From these numbers it is seen that the ratio of the quantities of material ppd. varies con- tinuously, and in the same manner as the ratio of the amounts of salts employed ; with equal masses of the two sulphates in solution the pp. contains much more nickel than manganese; hence it is at once inferred that the basic power of manganous hydrate or oxide is greater than that of nickel, since the less basic a material the greater its tendency to be affected by the pptant. Extending these experiments performed in a similar manner to mixtures of nickel and cobalt sulphates, but employing sodic hydrate instead of carbonate, it was found that these two salts have almost equal degrees of precipitability; that is to say, if the two salts exist in the solution in equal amounts they will accumulate in the pp. in about equal quantities; or, with varying quantities of material, the ratio of the amounts of the two salts ppd. will be approximately equal to the ratio initially in the solution ; in other words, the basic powers of the two salts are about equal. (For the theory of fractional pptn. see Hood, P. M. 1886.) Reduction of Oxides. The conditions that affect the reduction of metallic oxides by hydrogen, carbon monoxide, and carbon, have been examined by Wright and Luff (O. J. 33, 1,509; 35, 475; 37, 757), the type of the reactions being represented by the equation a + bc = ab + c. The results have important practical bearings on metallurgical operations. The temperature at which reduc- tion commences is a function of (1) the physical conditions of the bodies experimented with, (2) and the chemical nature of the substances. With CO as the reducing agent, the temperature at which action begins in the case of cupric oxide varies from 60° to 146° according to the state of aggregation of the copper oxide; for ferric oxide the temperature ranges between 90° and 220°. The reduction by CO of copper oxide, prepared by ppn., is well marked at temperatures below 100°, but at 100° it becomes very ener- getic. The initial action of H on copper oxide was found to take place at temperatures ranging between 85° and 172°, and on ferric oxide be- tween 195° and 265°. When carbon was em- ployed as the reducing agent, the temperature of initial action varied not only with the physical nature of the metallic oxide, but also with the quality of the carbon ; the temperature limits for copper oxide were 350° and 440°, and for ferric oxide 430° to 450°. By comparing the tempera- tures of initial action for a given kind of me- tallic oxide, it was invariably found that that reducing agent begins to act at the lowest tem- perature which has the greatest heat of combus- tion, so that the heat disturbance during its action has (algebraically) the greatest value. Thus H always begins to act at a lower tempera- ture than carbon, and CO at a lower temperature than H,as the following table shows for different specimens of metallic oxides :— CO H Sugar C C from CO Cupric oxide a . 60° 85° 390° 350° ,, B . 125 175 430 350 ,, c . 146 172 440 430 Cuprous oxide 110 155 390 345 Ferric oxide a . 202 260 450 430 ,, B . 90 195 450 „ C . 220 245 450 430 Comparing cupric and ferric oxides prepared by analogous processes, and therefore pre- sumably in much the same physical state, it was uniformly found that the temperature of initial action of a given reducing agent is lower on oxide of copper than on oxide of iron, as the following numbers show](https://iiif.wellcomecollection.org/image/b21995990_0001_0767.jp2/full/800%2C/0/default.jpg)