Volume 1
Watts' dictionary of chemistry / revised and entirely rewritten by H. Forster Morley and M.M. Pattison Muir ; assisted by eminent contributors.
- Date:
- 1888-1894
Licence: Public Domain Mark
Credit: Watts' dictionary of chemistry / revised and entirely rewritten by H. Forster Morley and M.M. Pattison Muir ; assisted by eminent contributors. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
769/796 (page 743)
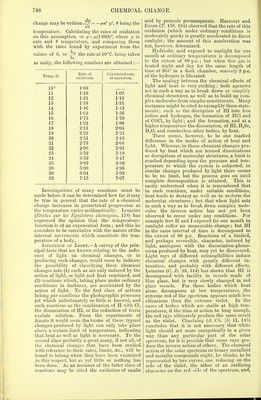
![product of the primary action could be deter- mined experimentally, by introducing a known mass of the body into the system, and com- paring the rate of the change with that observed when no more of the specified body was present than was formed during the primary reaction. Since ak is the member of the system whose rate of change is the object of measurement, let the amount that remains unchanged at time t, that is a< — aK, be taken as y ; then—if the initial quantities of the other members be cp v2, vu equivalents of A„-A, = e^A., ki = e2v2k„ a„ = 6ncnAx, and a, =e,a^, a., = e.2aK, an = flla„. In- serting these values in the above equation it becomes In this equation // and u' are constants to be determined experimentally, a being the initial value of y ; ft' is proportional to the rate and is dependent on the temperature (v. influence of HEAT ON CHEMICAL CHANGE, p. 741). Numerical examples of this equation for a system comprising the three bodies, ferrous chloride, hydric chloride, and potassic chlorate, have been given by Hood (P. M. [5] 20, 444), but the solutions he employed were so dilute that the products of the action appeared to influence the rate inappreciably, consequently the term in the equation relating to these effects was neglected, and the equation was taken as : -%=*'V( + + - (3) for the system of three bodies. It is possible to arrange the experimental conditions in such a way that, neglecting the action of the products, the course of the change may be much simpler than is represented by equation (2). This may be done, (1) by having all the active substances present in very large excess over that one which is made the object of measurement, so that they undergo but slight diminution between the beginning and the finish of the change taking place in the body measured; or (2) by arranging the constituents so that one or more of them, although taking part in the reaction, remains constant in amount, one con- stituent only diminishing in value. The equa- tion for the rate of change of one member in either case would be by (2) dy Where A,, a,,...a„ are the masses of the chemically active constituents which remain constant or nearly so ; or integrating, y = -Be'a', a being equal to jUAp a,...a„. Harcourt and Esson (T. 157, 117) proved the truth of this exponential formula for the action between a soluble iodide and hydric peroxide. The fundamental change in this case is represented by H20.> + 2HI = 2H..0 +12. By the simple device of adding a known con- stant amount of sodic thiosulphate to the active solution each time the liberated iodine made its appearance, the amount of hydric iodide was kept constant, while the H202 alone dimi- nished. The successive additions of thiosulphate measured the amount of change of the hydric peroxide (or y), and the intervals between each addition, or rather the appearances of free iodine, measured the times of action. From then- experiments relating to the influence of variations of temperature, and variations of the masses of the acting substances, Harcourt and Esson con- cluded that ' whether the solution contains in lc.c. 74(5 milhonths of a gram of hydric sulphate or 150 times that quantity, 604 millionths of a gram of KI or 9 times that quantity, or whether HC1 or hydric sodic carbonate be substituted for H2S04, whether the temperature be 0° or 50°, and whether the portions of change require for their accomplishment intervals of one or two minutes, or intervals of half an hour or an hour, this reaction still conforms to the law that the amount of change is at any moment proportional to the amount of changing substance.' Harcourt and Esson (T. 156, 193) had pre- viously employed the reaction between potassic permanganate and oxalic acid for investigating the laws according to which a chemical change progresses. Although this investigation was not quite successful in its primary object, it serves well to illustrate the anomalous results that may be obtained by the interfering action of the pro- ducts formed in a reaction, or by extraneous salts. The reaction under examination may be represented at its beginning and its conclusion by the two sides of the equation: K.,Mn.,Os + 3H,S04 + 5H,C,04 = K2S04 + 2MnSO, + 10CO2 + 8H20. The reaction progresses with moderate rapidity at temperatures easily kept under control. By varying the mass of any one of the constituents a corresponding variation occurs in the rate of oxidation. The influence of H2S04 is shown in the following table; the reaction was allowed to go on in each case for four minutes, and was then suddenly stopped by the addition of KI, the amount of change that had taken place being obtained by estimating the iodine liberated:— Mole- cules H2S04 Per cent, change in 4 min. Molecules H..SO., Per cent, change in 4 min. 2 21-8 10 71-6 4 36 12 77-4 6 51-1 14 82-1 8 635 16 85-7 22 92-3 The principal secondary reaction in the oxida- tion of C2H20,| by K,Mn,Os arises from the de- composition of K2Mn208 by the MnSO, (K.Mn.Oe + 3MnS04 + 2H..0 = K,S04 + 2H,S04 + 5MnO,); this reaction in- fluences the rate of oxidation in a remarkable manner. With the materials in the proportions of K,,Mn2O8:10H2SO4:5H,C.,O4, it was found that when no manganous sulphate was added only eight p.c. of chemical change took place in 4 mins., but by gradually increasing the mass of MnS04 the amount of change taking place in this interval of time increased, until it reached 85 p.c. when 3MnS04 was present. Further in- crease of the MnS04 only slightly altered the rate of oxidation. Harcourt and Esson likewise found that by varying the masses of H,S04 and C2H.,04, the K,Mn208 and MnS04 remaining constant, the percentage of chemical change in a definite time (3 mins.) gradually increased till it reached](https://iiif.wellcomecollection.org/image/b21995990_0001_0769.jp2/full/800%2C/0/default.jpg)