Volume 1
Watts' dictionary of chemistry / revised and entirely rewritten by H. Forster Morley and M.M. Pattison Muir ; assisted by eminent contributors.
- Date:
- 1888-1894
Licence: Public Domain Mark
Credit: Watts' dictionary of chemistry / revised and entirely rewritten by H. Forster Morley and M.M. Pattison Muir ; assisted by eminent contributors. Source: Wellcome Collection.
Provider: This material has been provided by the Royal College of Physicians of Edinburgh. The original may be consulted at the Royal College of Physicians of Edinburgh.
773/796 (page 747)
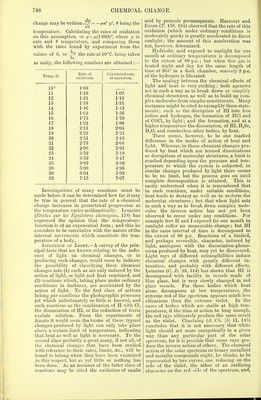
![ing to the theory of mass) react upon each other per minute in a solution kept of normal strength. The formula could be written as a = a + Bt'1, indicating the rate to vary nearly as the square of the temperature. Menschutkin (J.pr. [2] 29, 437) employed three somewhat analogous reactions for the study of this subject; (1) formation of ethylic acetate from acetic acid and ethylic alcohol, (2) formation of acetanilide from acetic acid and anihne, (3) formation of acetamide from acetic acid and ammonia. Molecular quan- tities of the materials were heated for one hour at different temperatures, and the amounts of ether, anilide, and acetamide formed were deter- mined, and taken as measures of the speeds. The following are his results : — Temp. Ether formed Temp. Anilide formed Temp. Aceta- mide formed 90 7-50 82° 6-08 100° 0 102 13-50 90 8-50 110 1-27 112 19-02 102 14-59 121 4-41 122 24-78 112 21-51 130 9-02 132 32-60 122 30-71 140 21-36 142 40-65 132 39-91 150 36-96 152 46-82 142 47-65 152 40-66 162 52-99 152 55-49 155 50-90 172 57-45 162 61-57 160 58-67 182-5 60-99 172 66-39 172 72-33 212-5 63-98 182-5 68-87 182-5 78-31 212-5 72-19 212-5 82-83 These numbers all agree in this respect, that the differences in the amounts of action during one hour, for equal differences of temperature, j gradually increase, pass through a maximum at a definite temperature, and then decrease. As regards the general inferences that might be drawn from these experiments relative to the connection between action of heat and rate of change, it must be remembered that the re- actions labour under the disadvantage of being cases of only limited action, and that the pro- ducts of the change no doubt retard the prin- cipal reaction, and tend to complicate matters. Besides this, the method of allowing the change to proceed in each case for the same interval of time is objectionable, for at the higher tempera- tures the amounts of the products formed before the expiration of one hour are so very much greater than the amounts formed at the lower temperature that their presence must exercise a considerable retarding influence on the further progress of the reaction up to the time-limit. Unlike some of the etherification processes the limits of formation of acetanilide and acet- amide are influenced very considerably by heat, as the following numbers show :— Acetanilide Acetamide Temp. Limit Tern]). Limit 100° 80-05 125° 75-10 125 83-11 140 78-18 135 82-39 155 81-46 145 81-22 182-5 82-82 155 79-08 212-5 84-04 182-5 78-85 212-5 77-75 In order to determine the temperature- function influencing the rate of a chemical change, Hood (P. M. [5] 20, 323) has again studied the oxidation of ferrous sulphate solu- tion by potassic chlorate. This reaction is well adapted for work of the kind, as it is com- pletely under control, and can be rendered as quick or as slow as may be desired by altering such conditions as dilution, temperature, amount of free acid, &c. The progress of the oxidation can also be followed with the greatest precision by means of permanganate. Each experimental solution consisted of -5637 gram of iron as ferrous sulphate, and 3-099 grams of free H,SO,, made up to a volume of 250 c.c. To this solution 10 c.c. of a solution of KC103 were added, equal to -2057 gram, being the oxidising equivalent of the iron. From such a solution, maintained at a constant temperature, 10 c.c. were withdrawn at indefinite intervals of time, and titrated by permanganate, and from several such observations the constants in the equation y(a + t) = b were calculated ; y being c.c. of permanganate, and t being time in minutes. Since 6 is inversely proportional to the rate of change, or chi = — ?L = — Uf(&)y-, by compar- ing the values of b obtained from a series of ex- periments in which everything remains the same except the temperature, a measure is obtained of the influence of heat on the rate of the oxida- tion, and consequently a means of finding the probable nature of the temperature-function f(S). The following table contains the results of Hood's experiments ; the values for a and b for the equation y(a + t) = b being the means of several experiments:— Temp. C. a b Ratio *5— bn+l 10° 330-8 3327-8 11 301-6 3025 1-100 12 274-7 2752-9 1-098 13 250 2503 1-099 14 227-5 2282-7 1-096 15 206-6 2055-7 1-110 16 194-3 1920-8 1-070 17 174-2 1733 1-109 18 159 1577-4 1-098 19 147-1 1452-6 1-086 20 134-4 1325-4 1-096 21 124 1216-8 1-089 22 114-9 1123 1-083 23 102-6 1002-3 1-120 24 94-8 924-5 1-084 25 89-9 869 1-064 28 68-5 654-8 1-099 30 5S-7 551-2 1-090 32 50-3 465-3 1-088 Mean , 1-093 From the numbers under ——?— it appears that this ratio has as nearly as possible a con- stant value, the mean of all the experiments being 1-093 : it would seem, therefore, that for this reaction at least the temperature-function has an exponential form, and that the rate of](https://iiif.wellcomecollection.org/image/b21995990_0001_0773.jp2/full/800%2C/0/default.jpg)