Departmental report : 1998 (MAFF) / Department for Environment, Food and Rural Affairs and the Forestry Commission ; presented to Parliament by the Secretary of State for Environment, Food and Rural Affairs.
- Great Britain. Department for Environment, Food & Rural Affairs
- Date:
- 1998
Licence: Attribution 4.0 International (CC BY 4.0)
Credit: Departmental report : 1998 (MAFF) / Department for Environment, Food and Rural Affairs and the Forestry Commission ; presented to Parliament by the Secretary of State for Environment, Food and Rural Affairs. Source: Wellcome Collection.
57/296 (page 46)
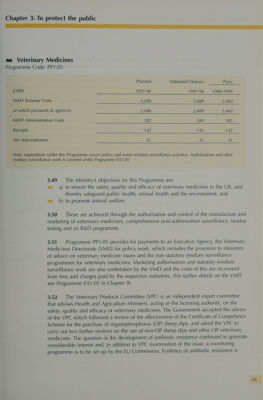
![ce ma Veterinary Medicines Programme Code: PP1:05 Planned Estimated Outturn Plans £'000 ) 1997-98 1997-98 1998-1999 MAFF Scheme Costs | 2,688 2,688 2,460 of which payments to agencies 2,688 2,688 2,460 MAFF Administration Costs 183 183 183 Receipts -142 -142 -142 Net Administration 41 41 4] Note: expenditure under this Programme covers policy and some residues surveillance activities. Authorisation and other residues surveillance work is covered under Programme EA1:05 3.49 The Ministry’s objectives for this Programme are: m= a) to ensure the safety, quality and efficacy of veterinary medicines in the UK, and thereby safeguard public health, animal health and the environment, and ea b) to promote animal welfare. 3.50 These are achieved through the authorisation and control of the manufacture and marketing of veterinary medicines, comprehensive post-authorisation surveillance, residue testing and an R&D programme. 3.51. Programme PP1:05 provides for payments to an Executive Agency, the Veterinary Medicines Directorate (VMD) for policy work, which includes the provision to Ministers of advice on veterinary medicine issues and the non statutory residues surveillance programmes for veterinary medicines. Marketing authorisation and statutory residues surveillance work are also undertaken by the VMD and the costs of this are recovered from fees and charges paid by the respective industries. (For further details on the VMD see Programme EA1:05 in Chapter 9). 3.52 The Veterinary Products Committee (VPC) is an independent expert committee that advises Health and Agriculture Ministers, acting as the licensing authority, on the safety, quality and efficacy of veterinary medicines. The Government accepted the advice of the VPC which followed a review of the effectiveness of the Certificate of Competence Scheme for the purchase of organophosphorus (OP) sheep dips, and asked the VPC to carry out two further reviews on the use of non-OP sheep dips and other OP veterinary medicines. The question of the development of antibiotic resistance continued to generate considerable interest and, in addition to VPC examination of the issue, a monitoring programme is to be set up by the EU Commission. Evidence of antibiotic resistance is](https://iiif.wellcomecollection.org/image/b31848758_0057.jp2/full/800%2C/0/default.jpg)