Researches on morphine. Pt. 1 / by S.B. Schryver and and Frederic H. Lees.
- Schryver, S. B. (Samuel Barnett), 1869-1929.
- Date:
- [1900]
Licence: In copyright
Credit: Researches on morphine. Pt. 1 / by S.B. Schryver and and Frederic H. Lees. Source: Wellcome Collection.
9/20 (page 1030)
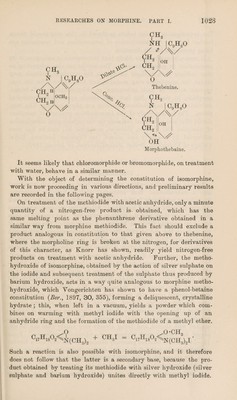
![standing gradually becomes hard, and can be readily purified by re¬ crystallisation. Chloromorphide is a beautifully crystalline product which melts with decomposition at 190°. It is scarcely soluble in ether, although, when freshly precipitated from a solution of its salts by alkalis, it dissolves readily in this solvent, but separates out again almost immediately in a brilliant, crystalline form. It is easily soluble in chloroform, and in methyl and hot amyl alcohols, but only sparingly so in boiling ethyl alcohol. It is insoluble in benzene, light petroleum, and most other organic solvents. 0-1707 gave 0*4214 C02 and 0-0917 H20. C = 67-32 3 H = 5*96. 0*1290 „ 0*3180 C02 „ 0*0715 H20. C = 67*22 ; H = 6*15. 0*2830 „ 11*8 c.c. moist nitrogen at 20° and 760 mm. N = 4*77. 0*3702 ,, by Carius’method, 0*1731 AgCl. 01 = 11*57. C17H1802NC1 requires C = 67*4; 11 = 5*93; Cl = 11*68; N = 4*61 per cent. A determination of the specific rotation in methyl alcohol gave the following result: aD= -2°9', 1=1 dcm., c = 0*573, [a]D= -375*2°. Chloromorphide Hydrochloride is prepared from the base by grinding it up in a mortar with a slight excess of 10 per cent, hydrochloric acid. A pasty mass is at first formed, but on grinding it is rapidly converted into a snow-white powder, which can be readily filtered off from the mother liquor. Its aqueous solution is unstable, for reasons already described, and it can only be recrystallised from water with considerable loss. It dissolves readily in hot alcohol, and on slowly cooling separates in hard crusts of diamond-like, highly refractive, anhydrous crystals. 0*2456 gave, by Camus’ method, 0*1969 AgCl. Cl =19*84. C17H1802NC1,HC1 requires Cl = 20*88 per cent. Its aqueous solution is very strongly lsevorotatory. ajf = -5°16', 1=1 dcm., c = l*67, [a]2D°°= -315*3°. The hydrobromide is prepared in the same way as the hydrochloride, and is similar in its properties. Neither in this salt nor in the hydro¬ chloride could the halogen acid combined with the base be determined by direct titration with silver nitrate, as the end reaction was not sharp, probably owing to the ease with which the salt is decomposed. The salt used for analysis was recrystallised from absolute alcohol. 0*3241 gave, by Carius’ method, 0*2716 of mixed AgCl and AgBr. Cl7H1802NCl,HBr requires 0*2793 gram. The specific rotation of the salt recrystallised from alcohol gave the following result :](https://iiif.wellcomecollection.org/image/b30599210_0009.jp2/full/800%2C/0/default.jpg)